Sang-Soo Lee, PhD, MBA, Sr. Director, Market Access and Government Affairs, Medtronic Korea, and Head of Center of Expertise (COE), Market Access and Government Affairs, Medtronic Asia Pacific, Seoul, South Korea
- MoHW announced “Measures to improve National Health Insurance (NHI) sustainability and support for essential medical care”
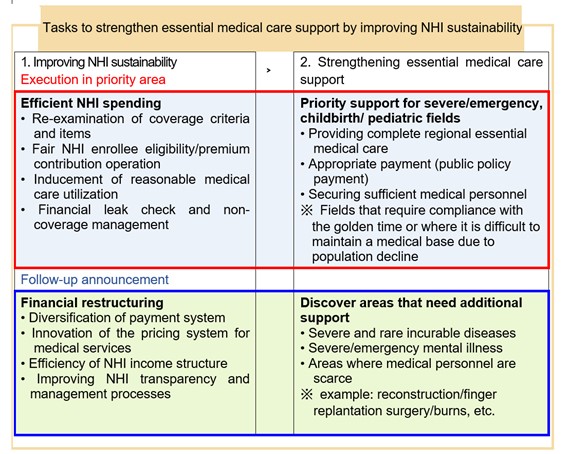
On December 8, 2022, the Ministry of Health and Welfare (MoHW) held a public hearing on “Measures to improve National Health Insurance (NHI) sustainability and support for essential medical care”.[1] MoHW launched the “National Health Insurance financial reform execution team” on August 23 and “Essential medical care expansion execution team” on August 25. The Ministry has been preparing measures to enhance the sustainability of NHI and restore the foundation for essential medical care. It has been pointed out that the extensive NHI coverage enhancement policy has a positive function of improving medical access but acts as a threat to NHI financial soundness by causing excessive medical care. Despite large-scale financial input, the imbalance in the healthcare delivery system, such as the concentration of large hospitals in the metropolitan area, has deepened, and efforts to support essential medical care directly related to the lives of people have been insufficient. In the future, the government plans to promote fiscal efficiency to ensure the sustainability of NHI while maintaining the NHI benefits that are being appropriately used by people. The finances saved through this effort will be used to support the essential medical care directly related to the lives of people and catastrophic medical expenses that are burdensome to people.
To maintain the sustainability of NHI amid an aging population and increasing medical demand, MoHW plans to enhance financial soundness and transparency. The "National Health Insurance sustainability improvement plan" announced at this public hearing includes re-examination of benefit items and criteria such as MRI/ultrasound examination, NHI enrollees’ fair eligibility management, rational care utilization inducement, financial leakage inspection, and non-coverage management, etc. In 2023, the “2nd Comprehensive National Health Insurance Plan (2024 through 2028)” will be established, and the plan will be presented to reform the NHI financial structure, such as diversification of payment systems, efficient financing, and improved transparency in financial management.
MoHW plans to promote expenditure structure reform to improve long-term fiscal soundness of NHI. To shift from a ‘volume-based’ payment into a ‘value-based’ payment system, MoHW plans to promote the introduction of a new alternative payment system in addition to the fee-for-service system. It plans to diversify the payment system, such as lump-sum payment by medical institution and network participation payment, through the operation of pilot programs in essential medical care. It also plans to promote the establishment of a NHI Innovation Center to launch various pilot programs and alternative payment systems by medical institution type (primary care to tertiary care hospitals) and patient group (children, and emergency patients, etc.).
Major pilot programs related to diversified payment systems
| No. | Pilot Program | Payment Method |
| 1 | Pilot program to strengthen the severe disease care system | Tertiary hospital performance-based payment |
| 2 | Pilot program for retrospective payment for Children’s public specialty care center | Performance-based retrospective payment |
| 3 | Pilot program to improve the emergency cardio-cerebrovascular delivery system | Secondary and tertiary hospital network payment |
| 4 | New-Diagnosis Related Group (DRG) pilot program | DRG and Fee-for-service |
| 5 | Primary care chronic disease management pilot program | Fee-for-service and Bundled Payment |
The budget saved through spending reform is planned to be properly invested in where it is absolutely necessary, such as essential medical care. The “Essential medical care support measure” is to build a “regional complete essential medical care system” that supports critical cases/emergency, childbirth, and pediatric patients with high priority to receive essential medical care 24 hours a day, 365 days a year, within the golden hour near patients’ residences. In the future, MoHW will continue to discover essential medical care fields that require support and prepare additional measures.
2) MoHW/MFDS announced three medical devices as innovative medical devices under the Parallel Review program
On December 15, 2022, the Ministry of Health and Welfare (MoHW) and the Ministry of Food and Drug Safety (MFDS) announced a total of 3 medical devices, including 2 digital therapeutics for insomnia improvement and 1 artificial intelligence (AI)-enabled cerebral infarction diagnostic aid software, as innovative medical devices under the Parallel Review program in medical devices associated with regulatory approval, determination of new health technology assessment (nHTA) eligibility, and nHTA implementation.[2] The Parallel Review program was designed on October 31, 2022 by improving related regulations so that innovative medical devices using artificial intelligence (AI), big data, and digital technologies can be used quickly in the medical field. The relevant ministries and agencies such as MoHW, Korea Health Industry Development Institute (KHIDI), Health Insurance Review and Assessment Service (HIRA), and National Evidence-based Healthcare Collaborating Agency (NECA) will simultaneously conduct a parallel review on following matters that were carried out individually and sequentially in the past: 1) designation of innovative medical device, 2) determination of nHTA eligibility, 3) Innovative HTA, etc.
At the end of October 2022, a total of 8 medical devices were applied for the Parallel Review program, and as a result of reviewing 7 products that met review requirements, a total of three products, which include two insomnia improvement digital therapeutics (manufacturer: Aimmed Corporation and Welt Corporation) and a cerebral infarction diagnostic aid software (manufacturer: JLK Co., Ltd.), were designated.
Among the products designated this time, JLK Co., Ltd.'s cerebral infarction diagnostic aid software, which has already received MFDS regulatory approval, will enter the medical field (3 to 5 years) as non-covered service and item starting from the end of January 2023 at the earliest after going through the promulgation of the innovative medical technology notification (30-day notice of rulemaking). Two insomnia-improving digital therapeutics will enter the medical field as non-covered service and item through the announcement of the innovative medical technology notification (30-day notice of rulemaking) at the same time as the MFDS regulatory approval is completed.
3) HIRA disclosed the cost effectiveness evaluation result (incremental cost-effectiveness ratio, ICER) of drugs submitted for economic evaluation for 15 years
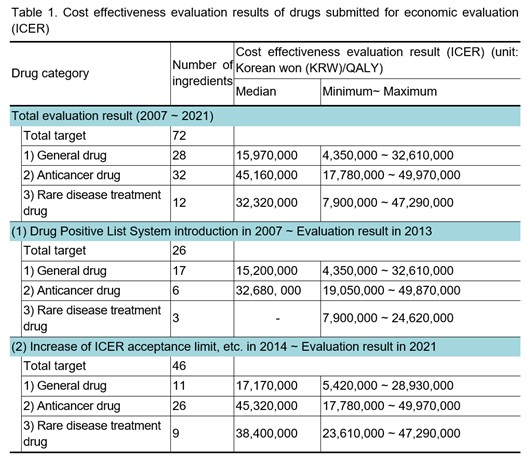
On December 16, 2022, the Health Insurance Review and Assessment Service (HIRA) disclosed the cost effectiveness evaluation result (incremental cost-effectiveness ratio, ICER) of drugs submitted for economic evaluation for 15 years (2007-2021) since the introduction of the Drug Positive List System for the first time.[3] ICER is a criterion for evaluating the economic feasibility of a new drug with improved effectiveness. It represents the increased effect of a new drug compared to a comparator or the additional cost per unit of utility. ICER interprets a new drug more cost-effective than a comparator if it is lower than a specific threshold. South Korea does not use an explicit threshold, and it is evaluated flexibly by referring to the existing deliberation results with the consideration of the severity of the disease, social burden of disease, impact on quality of life, innovativeness, etc.
This disclosure is due to the deletion of the 'GDP per capita' criterion and the addition of the 'existing review result' criterion in the revised ICER threshold-related regulation (in September 2021) of "the detailed evaluation criteria for negotiation subject drugs including new drugs." It has the meaning of an alternative reference value in South Korea, where the explicit threshold is not used. Starting with the first disclosure this year, evaluation results for the previous five years will be disclosed every December from now on. The number of ingredients for each drug category to prevent the evaluation results of individual drugs from being specified will be taken into account. In this disclosure, in the case of evaluation results of rare disease treatment drugs (three target ingredients) from 2007 to 2013, the minimum and maximum values are disclosed, but the median value is not disclosed to prevent the evaluation results of individual drugs from being specified.
Ingredients subject to disclosure shall be the ones that have been reviewed for cost effectiveness through economic evaluation by the ‘Drug Reimbursement Evaluation Committee’ and have been evaluated as coverage. Only in 2022, the entire evaluation results from 2007 to 2021 are disclosed. Previously, starting from 2014, major policy changes such as raising the ICER acceptance limit to strengthen coverage for severe diseases (in November 2013) and introducing the risk sharing program (in December 2013) were considered. Therefore, the evaluation results of 1) 2007-2013 and 2) 2014-2021 are disclosed together.
Drugs are classified into three categories: general drugs, anticancer drugs, and rare disease treatment drugs, and the number of ingredients and cost-effectiveness evaluation results for each drug category are disclosed.
References
1. http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=373999&utm_source=sfmc&utm_medium=email&utm_campaign=&utm_term=USKBC+Weekly+Newsletter+%e2%80%93+December+2-8%2c+2022&utm_content=12/8/2022
2. http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=374096
3. https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=10774&pageIndex=1