Objective
The goal of this working group is to address the current knowledge gap and provide guidance to the wider health economics and outcomes research (HEOR) and HTA communities on the concept of the living, dynamic phase of HTA. Specifically, the working group will:
- Provide a consensus definition of the “living HTA” approach that distinguishes it from similar terms, eg, lifecycle and adaptive HTA
- Present the main steps in building a living HTA approach
- Recommend possible good practices for implementing these steps
Rationale
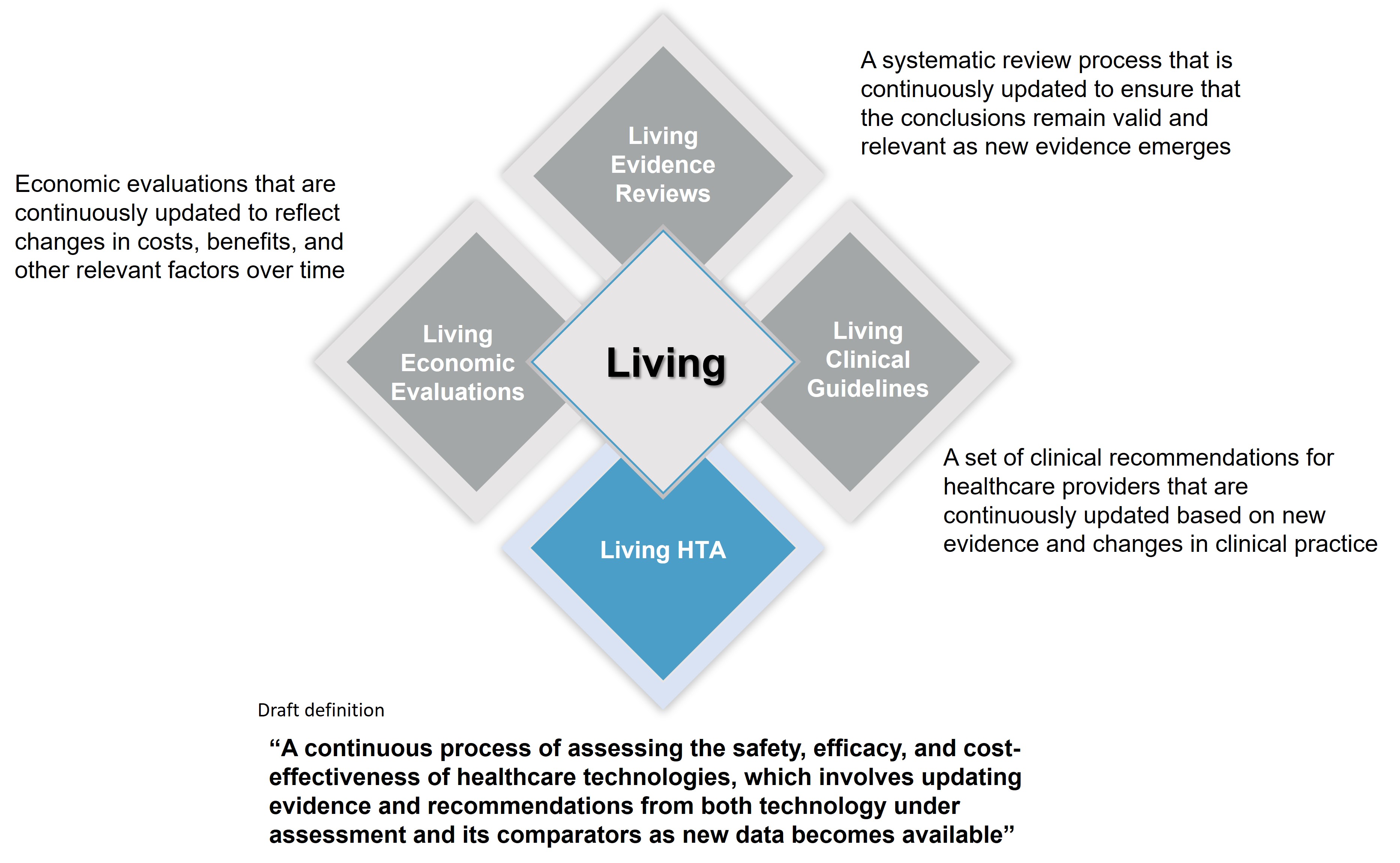
The living concept is already implemented within clinical guidelines (living clinical guidelines) with significant benefits for both healthcare systems and patients.
However, given the procedural differences between the development of clinical guidelines and HTA process, the former (living) model needs some adjustments before it can be directly implemented for HTA purposes (Figure 1).
Figure 1. Current Living Formats in Healthcare Decision Making
This working group will expand on pre-existing conceptual frameworks for lifecycle HTA processes (1) but will explicitly outline any differences between the two concepts and the methods for implementing a living HTA process. This includes foundational processes on how to safely and transparently integrate digital tools and AI in this process, acknowledging recent HTA efforts to provide clarity on the artificial use in evidence generation and reporting (2). For instance, eg, registration of digital tools and considerations for using AI, reporting standards to demonstrate validity and reliability, the need for checklists to ensure transparency in data collection and documentation, how often the review will be initiated and whether it is at a pre-defined timepoint or triggered by new evidence will be explored. In addition, reporting guidance (modifications to conventional HTA processes) on how these processes can be consistently implemented across disease areas and technologies will be discussed.
Some work has already happened in this area. The “Vienna Principles,” set up by the International Collaboration for the Automation of Systematic Reviews (ICASR), outlines the basic principles for 1) automation across the spectrum of review tasks, 2) continuous improvement and 3) how their integration can adhere to high-quality standards. An extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement for living systematic reviews highlighted how the use of automation during systematic reviews should be documented (3).
Recently, a review of NICE economic models outlined its main shortcomings around structural and methodological issues. It also highlighted how artificial intelligence (AI) could be used so that HTA agencies may proceed with regular and consistent technology re-appraisals, with the aim of exploiting potential updates in clinical effectiveness data, or substantial improvements in the structure and design of economic models (4). Recent examples of automated analysis and reporting for health economic modelling using open codes have been proposed (5).
- Kirwin E, Round J, Bond K, McCabe C. A Conceptual Framework for Life-Cycle Health Technology Assessment. Value Health. 2022 Jul 1;25(7):1116–23.
- National Institute for Health and Care Excellence. Position statement on the use of AI in evidence generation. [cited 2024 Oct 31]. Available from: https:https://www.nice.org.uk/about/what-we-do/our-research-work/use-of-ai-in-evidence-generation--nice-position-statement.nice.org.uk/about/what-we-do/our-research-work/use-of-ai-in-evidence-generation--nice-position-statement
- Extension of the PRISMA 2020 statement for living... | F1000Research [Internet]. [cited 2023 Apr 28]. Available from: https://f1000research.com/articles/11-109
- Daly MJ, Elvidge J, Chantler T, Dawoud D. A Review of Economic Models Submitted to NICE’s Technology Appraisal Programme, for Treatments of T1DM & T2DM. Front Pharmacol [Internet]. 2022 [cited 2023 Apr 28];13. Available from: https://www.frontiersin.https://www.frontiersin.org/articles/10.3389/fphar.2022.887298.3389/fphar.2022.887298
- Living HTA: Automating Health Economic ... | Wellcome Open Research [Internet]. [cited 2023 Apr 28]. Available from: https://wellcomeopenresearch.org/articles/7-194
Co-Chairs:

Seye Abogunrin, MSc, MPH

