ISPOR Science Strategy
Introduction
 The need has never been greater for health economics and outcomes research (HEOR) to inform healthcare decisions. Healthcare’s myriad challenges—the COVID-19 pandemic, the growth of innovative (but expensive) curative therapies, the move toward universal healthcare, the need to provide optimal health outcomes for patients within budget constraints, to name a few—combined with the increasing complexity of healthcare—bring the demand for HEOR to the forefront...
The need has never been greater for health economics and outcomes research (HEOR) to inform healthcare decisions. Healthcare’s myriad challenges—the COVID-19 pandemic, the growth of innovative (but expensive) curative therapies, the move toward universal healthcare, the need to provide optimal health outcomes for patients within budget constraints, to name a few—combined with the increasing complexity of healthcare—bring the demand for HEOR to the forefront...
Read More From the Introduction...
HEOR, as a blend of social, healthcare, and data sciences, provides a framework that can clearly define healthcare issues and generate the relevant evidence to inform healthcare decision making. Given today’s healthcare challenges, HEOR has the potential to markedly improve societal well-being and make a real impact in the lives of patients. To fulfill this opportunity, however, the field of HEOR will need to focus its efforts to navigate the variety and complexity of these challenges.
The objective of the ISPOR Science Strategy is to identify a targeted set of topical “themes” that the Society believes will have the greatest impact on both the field of HEOR and global healthcare. This Science Strategy ties directly to ISPOR’s mission to promote HEOR excellence to improve decision making for health globally. It also connects strongly to ISPOR’s Strategic Plan Update 2024 that prioritizes “continuing development of good research practices, providing platforms for dialogue, and producing highly regarded education and publications related to new and existing methods, technologies, and emerging trends in healthcare.” Implementing this effectively requires a directed Science Strategy, which is presented here as a complement to the Society’s overarching Strategic Plan.
ISPOR believes that this Science Strategy, when well executed, will help meet the evolving needs of healthcare decision making. The Science Strategy provides direction in 8 different themes of HEOR science and application. It covers most key areas of HEOR work, while calling out specific areas of focus in each theme. It is also important to note that while the Science Strategy is expected to define the greater part of ISPOR’s coming activities, new ideas are always welcomed and will certainly arise.
The development of the ISPOR Science Strategy was heavily informed by its members. As a member-driven organization, the Society relies on member input to guide all that it does. The first step in development of the Science Strategy was to gather topic suggestions by conducting a comprehensive survey of the members of the Society’s various groups, including its councils, special interest groups, board of directors, regional and student chapters, current and former task forces, etc. This initial survey resulted in 908 suggestions from members of 122 member groups representing more than 45 countries. The subsequent work to synthesize and summarize these research topics was led by ISPOR’s most senior advisory body, the Health Science Policy Council. Those methods are detailed in the appendix to this document. ISPOR is most grateful to its membership for the robust response to this effort. A listing of all the groups who responded is noted in the acknowledgements. Members’ enthusiasm for the progressing the science of HEOR is clear.
The ISPOR Science Strategy is a multiyear blueprint that will guide the Society’s efforts and mission-focused initiatives. As the leading professional society for HEOR globally, ISPOR’s intention is to leverage this Science Strategy to advance the science, drive innovation in the field, and have an even greater impact on improving healthcare decisions.
.Download the ISPOR Science Strategy
Science Strategy Themes
Real-World Evidence
Make HEOR evidence based on real-world data credible and reliable for use in healthcare decision making.
The growing abundance and richness of real-world data (RWD) in healthcare systems around the world has great potential to help improve the availability and delivery of healthcare by shedding light on many treatment-relevant questions that have not been—or cannot be—answered in randomized controlled trials (RCTs). However, this potential for the use of RWD remains limited due to concerns about data quality, ability to discern causality, and the transparency and reproducibility of the research process. While excellent progress has been made in each of these areas by health economics and outcomes research (HEOR) and scientists using RWD, that progress has not yet led to good practices becoming common practices, or to a broader understanding of under what circumstances, and for what purposes, evidence derived from RWD can be trusted. In this regard, progress can be enhanced by some specific efforts by ISPOR members and their collaborators.
- Develop criteria for evaluating and disclosing the research readiness of real-world databases for HEOR purposes, including the reliability of linkages across databases that improve their research capabilities. In some areas where gaps exist, particularly in low- and middle-income countries (LMICs), the efforts should focus on developing new RWD assets.
- Conduct further research into which—and under what circumstances—causal inference techniques, including those using machine-learning approaches, can reliably replicate or predict RCT results, and when they can be used to extrapolate beyond RCT results.
- Enable (through improved infrastructure) and incentivize researchers to follow good practices more routinely for RWD study protocol registration and reproducibility.
Why Real-World Evidence Matters
Real-world evidence (RWE) uses RWD—or data that are obtained outside of the context of randomized controlled trials (RCTs) to understand questions related to performance of technology or services in clinical practice. While RCTs randomly assign people to receive one of several healthcare interventions in a rigidly controlled study, RWD is usually generated either through observational studies and registries or through data collected during routine clinical practice. Examples include electronic health records; billing and claims data; registries; health apps and digital devices; patient-reported outcomes; and social media, with comments from patients and providers about product use and side effects. A more detailed definition of RWE can be seen in ISPOR’s “Good Practices for Real-World Data Studies of Treatment and/or Comparative Effectiveness: Recommendations From the Joint ISPOR‐ISPE Special Task Force on Real-World Evidence in Healthcare Decision Making.”
The intent of using RWE powered by RWD is not to replace RCTs, but to complement them. RWE can provide valuable information about how a drug, medical device, medical procedure, or other healthcare intervention can be expected to perform in the real world, in a way that RCTs will never be able to do. In addition, RWE can often be generated much more quickly and inexpensively than RCTs.
The use of RWE has its challenges—and challengers. Commonly voiced concerns about RWD studies include uncertainty about their internal validity, inaccurate recording of health events, missing data, and incomplete reporting of study conduct and analysis results. Critics of such studies also worry that available RWE results can be biased due to possible “data dredging” (ie, conducting multiple analyses until one provides the hoped-for result) and selective publication (ie, journals’ preference for publishing positive results).
ISPOR and its members are actively engaged in RWE and exploring better, more accurate methods to generate RWD. The Society has made RWE the topic of numerous presentations at its global conferences. Searchable content from ISPOR’s conferences session presentations, as well as citable research abstracts, can be found in the ISPOR Presentations Database. In 2019, the US FDA released its guidance on RWE, “Submitting Documents Using Real-World Data and Real-World Evidence to FDA for Drugs and Biologics Guidance for Industry.” This guidance cites a number of ISPOR Good Practices Reports on RWE.
ISPOR is emphasizing efforts to improve standards and practice for the collection and analysis of RWD and planning of analysis and reporting of RWE through its Real-World Evidence Strategic Initiative. This initiative is a critical part of ISPOR’s mission to promote HEOR excellence to improve decision making for health globally. Under this initiative is the Real-World Evidence Transparency Initiative, a collaborative effort between ISPOR, the International Society for Pharmacoepidemiology, the Duke-Margolis Center for Health Policy, and the National Pharmaceutical Council. The Initiative’s objective is to establish a culture of transparency for study analysis and reporting of hypotheses evaluating RWE studies on treatment effects.
ISPOR has also published a number of Good Practices Reports on Real-World Data that include recommendations for areas such as study registration, replicability, and stakeholder involvement in RWE studies; improving the reproducibility, rigor, and confidence in RWE generated from healthcare by establishing greater transparency about operational study parameters that are used to create analytic datasets from longitudinal healthcare databases; how to help policymakers recognize the benefits, limitations, and methodological challenges in using RWD; and a checklist of 27 questions that can be used to guide decision makers when they review retrospective database studies.
By encouraging adherence to existing methodological good practices, ISPOR endeavors to increase trust in the RWE generated by the global HEOR community as well as trust in, and acceptance of, economic models relying on RWE tools, technology, and core data models.
Economic Evaluation Methods
Stimulate methodological innovation in health economic evaluation and value assessment to better capture the complexities of new technologies and healthcare processes.
The core methods of health economic evaluation are generally well established but may not reflect some important considerations that enter into real-world decision making. For example, there are important insights from behavioral economics; more complex clinical pathways with treatment sequences and other trajectory modifiers; societal values that embrace dimensions beyond those in the standard quality-adjusted duration of life; and novel therapies that may be curative. In addition, understanding of the importance of variation—due to heterogeneity or residual uncertainty, including structural—has advanced, but methods for factoring into such evaluations are just beginning to develop. Approaches to estimating disease costs and budget impacts can also benefit from better delineation of these considerations, as well as from utilization of novel sources of real-world data (RWD). ISPOR’s mission includes the pursuit and support of advances in health economic evaluation that are relevant for decision making and the establishment of standards for their use when appropriate. Priorities for further research include methods that:
- Improve how economic analysis can broaden the capture of elements of value beyond the quality-adjusted life-year (QALY), as well as broader societal costs (eg, as shown in the Second Panel on Cost-Effectiveness in Health and Medicine’s Impact Inventory).
- Recognize and address heterogeneity of treatment effect and data limitations on outcomes measurement, including small sample sizes, confounding, and patient and clinician behavior.
- Address complex clinical pathways, including (1) treatment sequencing; (2) care pathways; (3) nonpharmaceutical interventions, such as telehealth; and (4) the impact of adherence.
- Advance both methods and platforms for open research, particularly health economic models
Why Economic Evaluation Methods Matter
Economic evaluation—such as cost-effectiveness analysis (CEA) and cost-benefit analysis—can play an important role in informing healthcare decisions by comparing costs and outcomes of healthcare interventions and assessing society’s willingness to pay for them. Despite more than 40 years of work in this area, many open questions and controversies still exist about its fundamentals, and new issues continue to arise that require innovative approaches to economic evaluation methodologies. The COVID-19 pandemic exacerbates the issue as this crisis has pressure-tested global healthcare systems, exposed more inequities in healthcare, and further revealed the limitations in the economic evaluation methods health economics and outcomes research (HEOR) professionals employ to address these issues. ISPOR’s mission to “to promote health economics and outcomes research excellence” obligates the Society to stay on the forefront of improving and extending the methods used for economic evaluation in healthcare.
One of the cornerstones of health economic evaluation—QALY—captures the principal benefits of treatment, namely improved survival and quality of life. However, the QALY has been criticized increasingly for not adequately capturing certain aspects that add to the benefit of a health technology, such as equity, risk aversion, and other personal and societal factors. The field of HEOR is gradually evolving methodologies to measure such factors and include them in the evaluation, however these advancements are very much a “work in progress.”
Economic evaluation has generally been oriented to assess a single health technology and an “average” patient. However, few patients are average and health service delivery usually combines services and technologies. To be useful in the real-world—particularly emerging situations such as providing more and more care by telehealth—economic evaluation must be adaptable and seen as relevant to more complex and nuanced decisions.
Traditional economic evaluation aims to produce the greatest cumulative health benefit in a population for a given amount of health spending, but it does not address how to distribute that health benefit within a population. Methods are developing to address these questions, but more work is needed as they are not yet well-known or much used.
ISPOR has a long history of setting standards and publishing Good Practices Reports in areas such as economic evaluation, health services delivery and process of care, and methodological and statistical research. These publications represent consensus guidance on the appropriate research methods, analysis, and reporting standards to conduct research to inform healthcare decisions and improve health. For topics with little or no published guidance, the Society established its Emerging Good Practices Reports to develop initial recommendations. These reports have covered a wide array of topics in economic evaluation including health economic modeling, CEA alongside clinical trials, budget impact analysis, dynamic simulation modeling in healthcare delivery research and, notably, evaluation of vaccination programs. A recent Special Task Force made recommendations for US Value Assessment Frameworks that included discussion of a number of elements of value that are not normally included in the QALY. ISPOR’s Good Practices Reports have been highly cited by researchers, used as part of educational curricula, and generally helped improve the rigor, reliability, and relevance of economic evaluation in healthcare.
The Society will be continuing to bring key stakeholders together—payers, policy makers, clinicians, and researchers—with a renewed focus on innovating health economic evaluation methodologies.
Patient-Centered Research
Refine the measurement of patient- (and proxy-) reported outcomes and preferences to improve their veracity, rigor, and usefulness for healthcare decisions.
Accurate and meaningful measures of patient-centered outcomes and preferences are critical to numerous decisions throughout product development, clinical research, regulation, technology assessment, and healthcare delivery. While there has been considerable progress in the last 2 decades in many disease and healthcare contexts, there are still major gaps in the availability of standardized clinical outcomes that are meaningful to patients and/or patient- (or proxy-) reported outcomes that produce evidence relevant for decision making. Such outcome measures are in turn necessary as a basis for health-state utilities required for economic evaluation, although not sufficient. Further research is needed to improve the methods for translating outcomes into utilities over the course of a disease or condition, particularly for special populations (eg, children, chronically disabled). Stated-preference research can be used to evaluate the relative importance of dissimilar treatment features and health outcomes and provide empirical evidence to inform value judgments. Application of these methods has significantly grown in recent years, but continued work is needed to enhance methods for elicitation and analysis. Specific areas of focus include:
- Contribute to the identification and development of standardized patient- (and proxy-) reported outcome measures within and across conditions that are meaningful to patients and useful to guide numerous types of decisions and establish their relationship to surrogate measures where needed.
- Develop an evidence base on the relative advantages, disadvantages, and properties of utilities generated by alternative preference elicitation and modeling methods.
- Refine, compare, and extend quantitative methods used to account for patients’ valuation of health in a way that facilitates generating quality-adjusted life years and other metrics for use in health technology assessment.
- Advance utility measurement, and its incorporation into economic evaluation, for populations and situations where standard approaches may not be appropriate (eg, children, disabled).
Why Patient-Centered Research Matters
Healthcare payers, providers, manufacturers, and policy makers have been talking about the “patient voice” for more than a decade, talk of which intensified since the United States Congress authorized the Patient-Centered Outcomes Research Institute (PCORI) in 2010. There is broad agreement that patients’ wants, needs, and preferences should be a primary driver in healthcare decision making. PCORI, a nonprofit, independent organization, provides research reports that strive to include the patient in all aspects of its work, including peer review. December 2020 marks the fourth anniversary of patient peer review of PCORI-funded research reports. Since the inception of the PCORI Peer Review Program, a patient peer reviewer has provided insights and recommendations on more than 98 percent of PCORI-funded research reports.1
There has been skepticism in the past about the formation of PCORI and its mission of comparative effectiveness research, along with fears that its decisions could come between physicians and patients.2,3 Today however, it seems obvious that patients’ preferences should be included in healthcare research. As the “end user” of healthcare products and services, most agree that patients’ input should be factored into research.
Over the past several decades, patient-centered research—specifically, patient health-related quality of life and stated preference studies—has increasingly become a more routine aspect of clinical research. However, while the concept of patient-centered research is now broadly accepted, in reality it is not being employed as ubiquitously or as early in the process as it should. It is still sometimes viewed as a “nice to have,” when in fact it is a “need to have.” For example, patient-centered studies could remove or reduce some of the burdens of participating in a clinical trial.4 Additionally, patient-centered clinical trial design could improve participant recruitment, study questions, and results dissemination.5
Deficiencies in the methodologies that are used to evaluate patient-reported outcomes (PROs) and preferences (and proxy-reported outcomes, such as clinician-reported outcomes) also exist. As an example, standard methodologies are not ideal to assess patient-reported preferences and outcomes for special populations, such as children and people with disabilities. For children, effective assessment requires developmentally sensitive conceptual models of child health and an appreciation for the rapid change in children’s cognitive capacities.6 And people with disabilities may have difficulty completing patient-reported outcome measures (PROMs) because of the cognitive demands,7 but redesigning the features of PROMs may mitigate the impact of those demands.
Patient-centered research is a topic at many of ISPOR’s scientific conferences. September’s ISPOR-FDA Summit 2020, "Using Patient-Preference Information in Medical Device Regulatory Decisions: Benefit-Risk and Beyond,” brought together stakeholders—FDA, health economics and outcomes research (HEOR) experts, patient groups, and medical device manufacturers—to determine the best ways to design patient-centered research studies that would yield data rigorous enough to go before regulatory bodies. There have been plenaries and other sessions related to patient-centered research at all of ISPOR’s global conferences.
ISPOR has a current task force and 2 special interest groups focused on patient-centered research. The objective of the Measuring Patient Preferences for Decision Making Task Force is to develop methodological guidance to design, implement, and interpret patient-preference studies that are more relevant to the needs of decision makers. The mission of the Patient-Centered Special Interest Group is to facilitate the involvement of patient representatives in all stages of research and decision making to improve healthcare, its delivery, and outcomes. The Health Preference Research Special Interest Group is focused on providing leadership to advance the development, implementation, and use of health preference research in support of health policy, the development of medical products, and patient care.
The Society has published more than 25 Good Practices Reports related to patient-centered research. Topics include PRO and observer-reported outcome assessment in rare diseases, pediatric PRO instruments to support product labeling, and addressing the factors that should be considered when selecting a mode or modes of PRO data collection in a clinical trial. Additional information on ISPOR’s work in patient engagement can be found on the Society’s Patient Engagement in HEOR webpage.
Special Populations and Technologies
Identify innovations and adaptations of health economics and outcomes research (HEOR) methods that target selected areas of disease management and decision making (eg, rare disease, precision medicine, etc).
Special populations and technologies are defined as those with unique features that pose particular challenges for evidence generation, health technology assessment (HTA), economic evaluation, and outcomes research. Examples include treatments for rare diseases, gene therapies, biosimilars, medical nutrition, and digital health monitoring, among others. In these situations, novel HEOR methods and approaches may be needed to inform decision making. HEOR research topics that focus on special populations and technologies include (but are not limited to) the following:
- Designs for prospective and retrospective studies to generate evidence for therapies used to treat rare diseases.
- Methods to evaluate the economic value of very high-cost or high-budget impact therapies that substantially benefit or cure disease.
- Novel payment models for very high-cost curative therapies (eg, gene therapies).
- Pricing and reimbursement for personalized medicine tests and biomarkers (companion diagnostics and standalone genetic or other diagnostic testing).
- Frameworks and best practices for evaluation and assessment of digital healthcare including remote delivery/monitoring and digital therapeutics.
- Best practices for comparing cost-effectiveness of biosimilars.
- Adaptation of HTA methods and processes and evidence generation to incorporate and evaluate nonstandard therapeutics outside of digital, including nutritional factors, alternative medicine, etc.
Why Special Populations and Technologies Matter
Traditional methodologies for HEOR and HTA often do not address special populations and technologies particularly well. One special population of note is patients with rare diseases, where often there are not sufficient numbers to effectively run a randomized controlled trial (RCT). In the United States, a rare disease is defined as a condition that affects less than 200,000 people. Additionally, ethical issues such as consent (for patients who cannot speak for themselves and do not have anyone to effectively speak for them) can make it difficult or impossible to run an RCT. This scarcity of data means that HEOR professionals need to broaden their approaches to generating an evidence base—with tactics such as collecting data outside of the traditional ways, using transparent RWE and systematic reviews, and adapting modeling methods—to compensate for the missing information.
Digital healthcare is another area that is exploding in growth and will require novel methods of HEOR assessment. In September 2019, AMCP’s Partnership Forum took a look at what evidentiary requirements are needed for third-party payers to cover digital therapeutics, and where they fit in pharmacy and medical benefits. Attendees agreed that the features that would need to be evaluated for coverage and formulary decisions include safety and efficacy, as well as the usability of the therapeutic.
As interest in biosimilars increases, so does the need to effectively assess costs. According to the authors of “Economic evaluation of biosimilars for reimbursement purposes—what, when, how?” published in the Journal of Market Access and Health Policy, typically when reimbursement is sought, an economic evaluation will not be necessary because the reference product is already reimbursed and is the standard of care.8 However, if that is not the case, then the biosimilar will need a full economic evaluation versus the standard of care.
ISPOR is routinely addressing these issues at its scientific conferences in sessions and posters, and in the Society’s flagship journal Value in Health. Recent articles include “Access and Unmet Needs of Orphan Drugs in 194 Countries and 6 Areas: A Global Policy Review With Content Analysis,” “A Review of Methodological Considerations for Economic Evaluations of Gene Therapies and Their Application in Literature,” and “PAM3 Hospital Nutrition Program Inform Cost Savings for Hospitalized Patients in Mexico.”
The Society’s Biosimilars Special Interest Group is identifying and discussing emerging issues of biosimilars, as related to their originator biologics, focusing on HEOR and reimbursement policy. The Digital Health Special Interest Group is addressing new opportunities in the healthcare sector emerging from the increasing use of digital technologies, specifically telemedicine and mobile devices (mHealth), and to evaluate the impact of information and communication technology on health outcomes.
ISPOR’s Nutrition Economics Special Interest Group is developing a systematic approach or specific methodology for the assessment of nutrition in outcomes research. The Oncology Special Interest Group is identifying and addressing specific oncology outcomes research issues with the goal of developing recommendations to address these issues. The Precision Medicine and Advanced Therapies Special Interest Group is disseminating information and providing leadership related to HEOR of precision medicine and advanced therapies. And the Rare Disease Special Interest Group is identifying issues in the rare disease environment so that all stakeholders can effectively address key challenges and more effectively establish the value of new and existing diagnostics and therapeutics..
HTA in Healthcare Decision Making
Identify approaches that strengthen the linkage between health technology assessment (HTA) and healthcare decision making to improve the efficiency, transparency, and fairness of both.
HTA has the potential to play an integral role in healthcare decision making. In practice, it often has less impact than expected. Strengthening the connection between HTA and healthcare decision making should enable HTA to reach this potential. Appropriate institutionalization of HTA, a greater degree of participation by different stakeholders, and improved transparency in the HTA process as well as the criteria used by the decision makers can improve the efficiency, transparency, and perceived fairness of this process. HEOR can help strengthen these linkages by investigating the following areas:
- Develop methods that influence universal health coverage and health system design, including addressing equity and disparities for medicines, diagnostics, preventive treatment, medical devices, and digital health technologies.
- Expand methods applied in decision models and decision making to account for differences such as cultural norms, socioeconomic status, and end-of-life preferences.
- Assess the reasons why variability occurs in healthcare decisions, including the role of key factors such as scientific evidence and the preferences of decision makers, and develop guidelines for decision makers to minimize variability based on that research.
- Develop methods and recommendations to inform rapid evidence synthesis and informed recommendations based on such evidence.
- Advance novel approaches to increase use of HTA to support decision making, including highlighting applicability of HTA to healthcare decisions and ensuring wider awareness and uptake of HTA and related analyses.
Why HTA in Healthcare Decision Making Matters
Health technology assessment (HTA) is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The purpose is to inform decision making in order to promote an equitable, efficient, and high-quality health system.9 HEOR is a key discipline involved in HTA. Countries with universal healthcare (UHC) systems tend to have a more systematic and centralized approach to healthcare evaluations using HTA compared to those without UHC—such as the United States.
HTA is critically important in informing healthcare decisions beyond any linkage to UHC. HTA has become a standard policy tool for advising decision makers who must manage the entry and use of pharmaceuticals, medical devices, and other technologies (including complex interventions) within health systems through reimbursement and pricing.
ISPOR has been leading the way with many HTA-related sessions at ISPOR conferences, summits, and webinars, and HTA-related articles in its journal, Value in Health. The Society also offers HTACentral.org, a microsite that is a comprehensive repository of resources and tools to support HTA.
ISPOR has published a number of Good Practices Reports on HTA. The Society published the ISPOR Report, “Identifying the Need for Good Practices in Health Technology Assessment: Summary of the ISPOR HTA Council Working Group Report on Good Practices in HTA,” in 2019. This publication was the first report in 20 years to comprehensively synthesize good practices in HTA. Notable in its collection of HTA-related Good Practices Reports are the Society’s “Multiple Criteria Decision Analysis for Healthcare Decision Making” Report 1—Introduction and Report 2—Emerging Good Practices. Additionally, ISPOR’s publication, “Challenges in Research and Health Technology Assessment of Rare Disease Technologies,” developed the first catalog of primary impediments to rare disease research and HTA.
ISPOR’s Joint HTAi - ISPOR Deliberative Processes for HTA Task Force is working to develop a consensus definition for a deliberative process from an HTA perspective and internationally recognized good practice recommendations on the use of deliberative processes in HTA. The Society’s HTA Council helps to identify areas of research focus like the deliberative processes efforts and organizes ISPOR’s HTA Roundtables, a platform to advance scientific methods; facilitate information sharing about the current state of HTA, its development, and its role in optimizing healthcare decisions; and to bridge the gap between technology assessors, private and public payers, regulators, and patients to improve health globally. The Roundtables convene annually in Asia Pacific, Europe, Latin America, Middle East and Africa, and North America.
Health Economics, Access, and Policy
Develop health economics and health technology assessment (HTA) tools that apply to health policy, research and development (R&D), pricing, and reimbursement to optimize and balance the needs of access, sustainability, and innovation.
Pricing and reimbursement are among the most visible and contentious issues in healthcare, especially for patent-protected drugs, devices, and diagnostics. Given patents’ and data exclusivity, how should products be priced to ensure the maximum access compatible with providing the maximum long-run health gains from innovation? Economic theory and incentives suggest—for optimal long-term production of innovation—that countries (and indeed individuals) should contribute based on their ability (and presumably willingness) to pay (ie, global differential pricing is called for) but how best to implement it? Furthermore, cost-effectiveness analysis (CEA), particularly but not only its use of quality-adjusted life-years (QALYs), remains a standard but much-debated approach to assessing value and determining prices; to better capture some aspects of value, a number of modifications to CEA have recently been suggested but need further exploration and testing. Separate from—but related to—pricing is coverage and reimbursement by a payer, where there is a wide variety of coverage paradigms across payers/countries as well as across types of drugs (eg, orphan drugs or accelerated approvals). Pricing and reimbursement decisions, generally made in the face of uncertainty about future benefits and costs, will not only affect patient access to innovative products, but also healthcare spending, which can raise sustainability questions. Research priorities that arise include:
- Address international pricing issues, including refinement and application of global differential pricing approaches that incentivize R&D globally—while promoting access in low- and middle-income countries (LMICs).
- Test the use of novel elements of value or of broader societal value considerations in value-based pricing in real-world situations to better understand actual willingness to pay by payer—and by implication, the citizens and patients they represent.
- Address uncertainty about effectiveness and/or budget risk by testing and investigating performance-based risk-sharing arrangements and other innovative pricing/coverage agreements.
- Explore how budget impact, affordability, and value for money are best addressed theoretically and empirically in general—and notably in the case of curative therapies—while considering the related issue of the efficient distribution of social value between innovators and payers over the entire product life cycle.
Why Health Economics, Policy, and Access Matter
Health economists know that serious inefficiencies exist in healthcare markets, whether these markets are publicly or privately run. These inefficiencies limit the ability of natural market forces to determine prices that optimally allocate resources to provide the products that patients value most highly. As a result, prices are often determined administratively in healthcare markets that are highly regulated. And unlike markets for consumer goods (such as cars) or services (such as auto repair), there is a lack of pricing transparency with many barriers that deter consumers’ ability to “comparison shop” for cheaper alternatives, particularly in an emergency.
In its application in HEOR, health economics has tended to focus on the total cost of care, in addition to measuring and valuing the outcomes of healthcare interventions to help inform pricing that reflects the patient and societal value of products. Health economics is also concerned with wider issues relating to the behavior of healthcare markets (for example, where and how resources are allocated, and who is using those resources); the performance of systems for funding (who pays for a drug or device or healthcare procedure, and how much do they pay); and providing healthcare including policy-oriented issues (such as reference pricing and intellectual property incentives).
However, health policy is not always well informed by HEOR. For example, HEOR has not played a large role in determining drug prices, even though demand for data about the true value of pharmaceuticals, especially for expensive new therapies, has been increasing every year. This pricing issue is not going to go away and continues to be considered by ISPOR. At the ISPOR Europe 2019 conference in Copenhagen, experts discussed the rationale for more modest drug pricing.10 ISPOR believes that better “linkage” of HEOR information and more clearly “interpreting” that research for policy makers would improve healthcare decisions.
While healthcare and healthcare decision making are so complex that it can be difficult to determine the best healthcare decisions without the appropriate tools, HEOR can provide the evidence to optimize decisions related to access and policy. Evidence-based policy and market access decisions improve the likelihood that patients will be able to obtain needed medications and other health technologies that will provide the best health outcomes within society’s ability and willingness to pay for them.
In its 2018 Value in Health article and Special Task Force report, “Defining Elements of Value in Healthcare—A Health Economists Approach: An ISPOR Special Task Force Report [3],” ISPOR introduced its “Value Flower,” which summarizes a dozen elements of value that should be considered in addition to QALYs gained, such as insurance value, the value of hope, and scientific spillovers. At the ISPOR Summit 2018, a follow-up to this Special Task Force work, the theme was “New Approaches to Value Assessment: Toward More Informed Pricing in Healthcare.” Summit sessions focused on both standard and more recently proposed approaches to measuring value and how they could be used in pricing and coverage decisions. The sessions also looked at the perspectives and decision contexts on value by patients, health plans, manufacturers, society, and others.
ISPOR continues the discussion of what kind of role HEOR can play in healthcare policy making in its conference sessions, summits, and webinars. In addition, the Society’s HTA Council and HTA Roundtables explore issues related to access and policy.
Low- and Middle-Income Countries
Promote health economics and outcomes research (HEOR) methods and resources that support health technology assessment (HTA) across the diverse range of health systems including low- and middle-income countries (LMICs).
HEOR methods are the cornerstone of HTA and value assessment. As more countries move toward a universal healthcare coverage model, prioritization within limited budgets is paramount. HEOR methods can be used and adapted in many different types of healthcare systems to help facilitate and align priority-setting across all aspects of a health delivery system, regardless of the stage of the country’s development. However, these methods are only helpful if there is synergy across problem definition, evidence generation, and decision making. Compounding this issue is that most HTA systems, structures, and standards are generally driven by developed countries. Thus, there is a need to better understand the applicability and sustainability of HEOR approaches especially for LMICs. ISPOR is well placed to address specific issues.
- Develop best practices for mapping and prioritization of assessment criteria through enhanced regional HTA collaboration efforts that focus on capacity building through training, engaging stakeholders, and advancing HEOR research aspects and publications within and across regions.
- Build regional adaptation capabilities through sharing and common usage of HEOR methodologies between developed and resource-challenged countries.
- Advance the methods involved in valuation of life across regions, including the assessment of preferences and selection of the valuation approach.
Why Low- and Middle-Income Countries Matter
LMICs often have very different healthcare needs and thus different HEOR focal points compared to countries with more developed healthcare systems. Many LMICs are moving toward universal healthcare (UHC) systems, making prioritization of healthcare services even more important. Additionally, basic public health issues, such as communicable diseases control and neonatal and maternal medical care,11 can be fundamental for LMICs. HEOR and HTA can contribute significantly to inform those public health decisions. From a more technical point of view, local and cultural considerations can play a role in some aspects of HEOR work—such as patient-reported outcome measures—and so adaptations may be necessary for country- or region-specific evaluations.
Many LMICs are in the process of developing HTA capacity in their countries. As such, some of the research and methods used to address issues in countries with fully developed HTA capacity may not be (or be as) applicable for LMICs. As an essay by Dr Lisa Hirschhorn, MD, MPH in BMJ Global Health noted, “The state of PHC [primary healthcare] research in low-income and middle-income countries (LMICs) is currently fragmented, uncoordinated and underfinanced.”12 Dr Hirschhorn further points out that most of the primary healthcare research in LMICs comes from a few high-performing middle-income countries, and entire regions of the world have little comparable and available data on primary healthcare systems, quality, and outcomes. In short, work is needed to determine what research can be adapted for use by LMICs and where new research and/or methods needs to be employed to support LMICs.
As part of its mission, ISPOR advances global HEOR excellence through its consortia, networks, and chapters. More than half of the Society’s regional chapters are based in LMICs. ISPOR commits significant support to its mission-focused initiatives with much of that devoted to LMICs. In 2020 alone, even during the impact of the pandemic, the Society invested US $2.7 million toward mission-focused initiatives. ISPOR’s chapters, networks, and consortia actively work with policy makers in their countries, with ISPOR, and with other markets to adapt HEOR research and to improve healthcare decision making in their geographies.
The Society also provides significant programming and education targeted to LMICs, offering conferences, webinars, short courses, and the ISPOR Health Technology Assessment Training Program. Membership fee waivers, ISPOR conference travel grants, and chapter educational funds are also provided to support members in LMICs. Chapter Leadership Training is also offered to support chapter leaders who are working to develop the science of HEOR in their regions.
Additionally, one of ISPOR’s peer-review journals, Value in Health Regional Issues, publishes articles from researchers who work in LMIC regions. The journal’s mission is to provide a forum for the advancement and dissemination of research that examines the use of healthcare resources alongside the clinical and economic health outcomes of patient populations in these regions.
Resilient Learning Healthcare Systems
Develop health economics and outcomes research (HEOR) methods and applications that contribute to resilient learning healthcare systems.
Healthcare systems are intertwined with many other aspects of how we live and how society functions—our economy, our environment, our demographics, our legal system, etc. The COVID-19 pandemic has highlighted the interconnectedness of health, public health, and the larger economy. To inform decisions that may have cross-sector ramifications, HEOR researchers should broaden their perspectives to include nonhealth-related outcomes, such as effects on the larger economy, educational outcomes, housing, and social welfare. To help healthcare systems learn, respond, and evolve effectively, ISPOR and its scientific agenda must be attentive to these broader issues, whether they are sudden—like the pandemic—or gradual, like demographic shifts and changes to the environment. We should also seek to collaborate with other disciplines that provide complementary perspectives and methods that will help us address these issues more comprehensively. Areas of focus in 2020 heavily related to COVID-19 and how interventions for contagious, potentially severe diseases can affect well-being in all dimensions, but we must be responsive to other issues as they arise. Given the nature of these concerns, ISPOR will seek collaborations with organizations in other disciplines when their participation would improve the ability to fully address the gaps identified below.
- Review the HEOR methods and data gaps highlighted by the COVID-19 situation and then create and prioritize an agenda for needed improvements.
- Assess the other known trends (eg, demographic shifts, environmental concerns, and others that may arise) to consider what HEOR methods and data are needed and what gaps may exist for analysis.
Why Resilient Learning Healthcare Systems Matter
LHSs are those in which research—in particular, evidence-based medicine—informs the continuous improvement of the healthcare system and patient care. The COVID-19 crisis has revealed that the need for resiliency in LHSs is now even more important so that healthcare systems can react and adapt quickly to emergent situations that affect healthcare.
Mullins et al (2018) wrote in the Journal of Comparative Effectiveness Research that the LHS model has 3 core components: an infrastructure for health-related data capture; care improvement targets; and a supportive policy environment.13 Contemporary technology and data support structures enhance an LHS’s capacity to collect and use data and evidence to measure, evaluate, and improve patient and public health. Care improvement targets help learning and health through clinical decision-making activities such as decision-support tools at the point of care, patient-centered care, and clinician-community links. And a supportive policy environment for an LHS includes financial incentives that reward high-value care, increased health system performance transparency, and buy-in from LHS leadership, healthcare providers, policy makers, and patients.
One example of this kind of system is Kaiser Permanente Washington’s Learning Health System Program that sees a key role of its program as helping its system to “act quickly to implement changes when urgent needs arise—such as the onset of the COVID-19 pandemic.”14 This LHS notes a number of highlights from their program that have helped its healthcare system combat the pandemic and related health issues.
HEOR has been focused historically on the micro level—for example, research about the health outcomes and/or economic impact of a new health technology. However, a more holistic and systems-based approach is needed from HEOR to have a truly comprehensive and durable impact on healthcare decision making—one that also informs decisions at the macro healthcare system level. In their article, Mullins et al describe a need for transitioning from LHSs to learning healthcare communities.13 They say a learning healthcare community (LHCC) model would combine the 3 core elements of an LHS with a fourth core component: active and continuous stakeholder and community engagement to improve the quality and value of healthcare within a community.
The COVID-19 pandemic has revealed inequities in healthcare systems, with minority and low-income populations suffering more intensely during the pandemic. When announcing $3.5 million in grant funding in April 2020 to attack racial disparities in the United States resulting from the pandemic, The Marguerite Casey Foundation noted that in Chicago, Black people represented 57% of all COVID deaths, even though they are only 30% of the population.15 Current HEOR methods and approaches are not currently well-suited for crises or shocks to the healthcare system.
Another factor expected to have a seismic impact on healthcare delivery is the aging global population. In places such as Japan, the graying of the population has already shown the stressors in play,16 as the number of working-age people who can support the system decreases and the number of older people who need higher levels of healthcare increase.
Climate change is also expected to exacerbate problems in healthcare systems. For example, climate change could contribute to more frequent and serious pandemics that will challenge even the most resilient LHSs. Extremes in the climate can also have other health consequences. For instance, the UN notes that climate change will increase the opportunities for malaria transmission in areas where it has been endemic, in areas the disease has been controlled, and in new areas that have not known the disease.17
The field of HEOR will be essential to successfully address these types of challenges and to provide the evidence that will inform resilient LHSs. Real-world evidence (RWE) will most certainly play a critical role in addressing the needs of informing LHSs. ISPOR’s significant work in this area is noted in this publication’s Real-World Evidence section. Additional information can be found on the Society’s Real-World Evidence Strategic Initiative webpage.
Appendix
References
1. Patient-Centered Outcomes Research Institute (PCORI). Patient Peer Review, PCORI Style. PCORI blog. https://www.pcori.org/blog/patient-peer-review-pcori-style. Published December 14, 2020. Accessed January 23, 2021.
2. Cannon MF. ‘The moment it produces useful CER, PCORI is toast.’ CATO at Liberty blog. CATO Institute. https://www.cato.org/blog/moment-it-produces-useful-cer-pcori-toast. Published January 3, 2012. Accessed January 23, 2021.
3. Basta N. Two cheers for PCORI. Pharmaceutical Commerce. https://www.pharmaceuticalcommerce.com/opinion/two-cheers-for-pcori/. Published November 10, 2019. Accessed January 23, 2021.
4. Science 37. Reaping the benefits of patient-centered clinical research. Trial Mix: Food for Thought. https://www.science37.com/blog/reaping-the-benefits-of-patient-centered-clinical-research/. Published February 9, 2018. Accessed January 23, 2021.
5. Heath S. 3 principles for patient-centered research design, clinical trials. PharmaNewsIntelligence. https://pharmanewsintel.com/news/3-principles-for-patient-centered-research-design-clinical-trials. Published December 2, 2019. Accessed January 23, 2021
6. Bevans KB, Riley AW, Moon JH, Forrest CB. Conceptual and methodological advances in child-reported outcomes measurement. Expert Rev Pharmacoecon Outcomes Res. 2010;10(4):385-396. doi: 10.1586/erp.10.52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3205357. Accessed January 23, 2021.
7. Schwartz AE, Kramer JM, Longo AL. Patient reported outcome measures for young people with developmental disabilities: incorporation of design features to reduce cognitive demands. Dev Med Child Neurol. 2018;60(2):173-184. doi: 10.1111/dmcn.13617. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5771952/. Published online November 24, 2017. Accessed January 23, 2021.
8. Moorkens E, Broux H, Huys I, Vulto AG, Simoens S. Economic evaluation of biosimilars for reimbursement purposes—what, when, how? J Mark Access Health Policy. 2020; 8(1):1739509. doi: 10.1080/20016689.2020.1739509. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144192/#__ffn_sectitle. Published online March 15, 2020. Accessed January 24, 2021.
9. International Society for Pharmacoeconomics and Outcomes Research (ISPOR). What is HTA? Health Technology Assessment Central. https://www.htacentral.org/what-is-hta/. Accessed January 23, 2021.
10. International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Has the time come for pharma to accept modest prices? https://www.ispor.org/heor-resources/news/view/2019/11/04/has-the-time-come-for-pharma-to-accept-modest-prices. Published online November 4, 2019. Accessed January 23, 2021.
11. Jakovljevic M, Getzen TE. Growth of global health spending share in low and middle income countries. Front Pharmacol. February 12, 2016. https://doi.org/10.3389/fphar.2016.00021. Accessed January 24, 2021.
12. Hirschhorn LR, Langlois EV, Bitton A, Ghaffar A. What kind of evidence do we need to strengthen primary healthcare in the 21st century? BMJ Global Health. 2019;4(suppl 8). http://dx.doi.org/10.1136/bmjgh-2019-001668. Accessed January 30, 2021.
14. Kaiser Permanente Washington Health Research Institute. Learning Health System Program. https://www.kpwashingtonresearch.org/about-us/capabilities/learning-health-system-program. Accessed January 25, 2021.
15. Margaret Casey Foundation. Margaret Casey Foundation Announces $3.5 Million in COVID-19 Grant Funding to Tackle Racial Disparities Resulting From Pandemic. https://caseygrants.org/who-we-are/inside-mcf/marguerite-casey-foundation-announces-3-5-million-in-covid-19-grant-funding-to-tackle-racial-disparities-resulting-from-pandemic/?gclid=Cj0KCQiA3NX_BRDQARIsALA3fIItvV-_aaG9AEoG2hPOWTSz-I3hFj93CsXnPZEDVNPwNKBSvsfhlCsaAkfaEALw_wcB. Published April 22, 2020. Accessed January 25, 2021.
16. Walia S. The economic challenge of Japan’s aging crisis. The Japan Times. https://www.japantimes.co.jp/opinion/2019/11/19/commentary/japan-commentary/economic-challenge-japans-aging-crisis/. Published November 19, 2019. Accessed January 30, 2021.
17. Fernando SF. Climate change and malaria – a complex relationship. UN Chronicle. https://www.un.org/en/chronicle/article/climate-change-and-malaria-complex-relationship. Accessed January 25, 2021.
Methods
ISPOR’s Science Strategy was created in a series of stages that included initial planning, solicitation of topic suggestions from all of the Society’s member groups, and final synthesis by a senior leadership group. The work proceeded as follows during 2020:
| January | The work plan was created by a small group of senior member leaders and staff. |
| February-March | Surveys were sent to all current members of ISPOR’s councils, committees, roundtables, and special interest groups and to current and past members of the Society’s task forces requesting their submission of 1 to 2 topic suggestions for the strategy. Similarly, the chairs of all regional and student chapters were asked to send the same survey request to all of their members. This survey effort resulted in 912 topic suggestions. The 912 original suggestions are posted separately on the ISPOR website. |
| March-April | For each group that submitted more than 10 topic suggestions from its members, the group leader was asked to synthesize the initial suggestions into a set of no more than 10 suggestions. This process resulted in a total of 544 topic suggestions from 118 groups. Response rates by eligible groups are shown in Table 1 below. All responding groups are listed in the acknowledgements. |
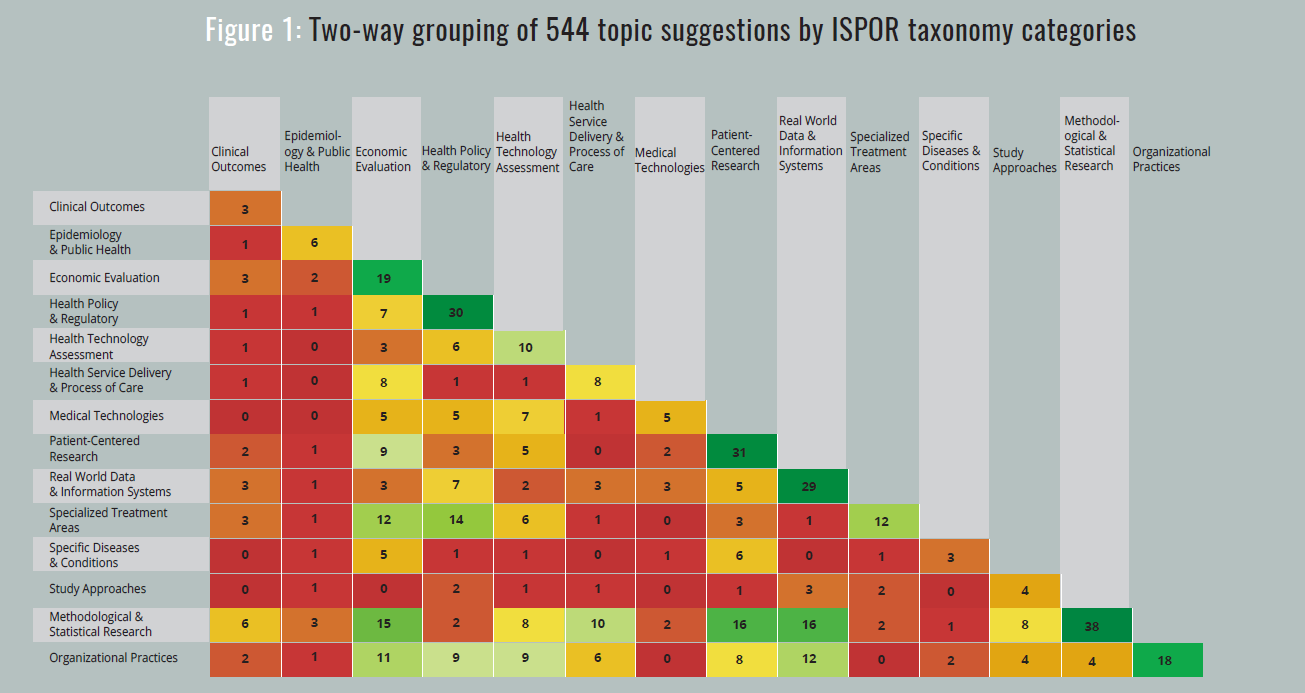
| April-May | ISPOR’s Chief Science Office staff classified the 544 suggestions into 1 or 2 categories of the ISPOR taxonomy. The resulting distribution of topics is shown in Figure 1. |
| May-July | The ISPOR Health Sciences Policy Council synthesized the 544 suggestions into 73 topics, based on similarity of topics and frequency of similar topics. |
| July | ISPOR’s Chief Science Office staff reclassified these 73 topics into 7 broad theme areas as a way to organize the strategic approach. |
| July-September | An extended Health Science Policy Council leadership group reviewed and discussed these theme areas and topics, resulting in the final synthesis with 8 theme areas, a description and rationale for each area, and 2 to 7 focused topics per theme area (34 overall) that comprise the strategy. |
| September-November | A subsequent ISPOR leadership and Board of Directors review resulted in modest changes in the final Science Strategy document. |
| Table 1 | |||||
| Group Type | Eligible Groups | Groups Responding | |||
| Special Interest Groups | 14 | 14 | |||
| Councils, et al | 12 | 12 | |||
| Task Forces | 19 | 18 | |||
| Regional Chapters | 85 | 41 | |||
| Student Chapters | 129 | 33 | |||
| Totals | 259 | 118 | |||
| Rate | 46% | ||||
| Rate w/o Students | 65% | ||||
Acknowledgments
ISPOR would like to acknowledge the substantial efforts of its Health Science Policy Council in the planning and execution of this Science Strategy initiative. The Society would also like to acknowledge the participation of the ISPOR groups listed below in providing the initial topic suggestions for the Science Strategy. Together, their contributions were responsible for the richness, diversity, and creativity of the topics that were the basis for this strategy.
Councils, Roundtables, and Committees
Awards Council
Board of Directors
Education Council
Faculty Advisor Council
Health Science Policy Council
Health Technology Assessment Roundtables
Institutional Council
New Professionals Network
Past Presidents Council
Patient Representatives Roundtables
Publication Committee
Short Course Committee
Special Interest Groups
Biosimilars
Clinical Outcome Assessment
Digital Health
Health Preference Research
Medical Devices and Diagnostics
Medication Adherence and Persistence
Nutrition Economics
Oncology
Open-Source Models
Patient-Centered
Precision Medicine and Advanced Therapies
Rare Disease
Real-World Evidence
Statistical Methods in HEOR
Task Forces
Budget Impact Analysis
Cost-Effectiveness Analysis in Clinical Trials
Consolidated Health Economic Evaluation Reporting Standards (CHEERS)
Clinical Outcome Assessment / Patient-Reported Outcome Health State Utilities
Indirect Treatment Comparison / Network Meta-Analysis
Machine Learning Methods in HEOR
Measuring Drug Costs in Cost-Effectiveness Analysis
ISPOR-Society for Medical Decision Making (SMDM) Modeling
Multiple Criteria Decision Analysis
Simulation Modeling and Optimization in Health Services Research
Real-World Data
Retrospective Database
Stated Preference Methods
Systematic Reviews With Cost and Cost-Effectiveness Outcomes
Economic Analysis of Vaccines
Value of Information
Regional Chapters
Argentina
Australia
Austria
Boston
Brazil
Chile
Chinese MDA-PE
Colombia
Croatia
Egypt
Ethiopia
India-Amaravati
India-Andhra Pradesh
Japan
Jordan
Kazakhstan
Kenya
Korea
Kuwait
Mongolia
New Zealand
Philippines
Russia
Russia HTA
Russia St Petersburg
Saudi Arabia
Shanghai
Singapore
Slovakia
South Africa
South China
Spain
Taiwan
Turkey
Uganda
Ukraine
United Arab Emirates
Uruguay
Uzbekistan
Venezuela
West China
Student Chapters
Auburn University
Danylo Halytsky Lviv National Medical University
Duquesne University
Florida A&M University
Javeriana University (Pontificia Universidad Javeriana)
Karolinska Institutet
Maastricht University
Massachusetts College of Pharmacy and Health Science
Monash University Malaysia
National College of Pharmacy
National University of Singapore
Rutgers University
Tehran University of Medical Sciences
TH Köln/University of Applied Sciences
Tulane University
Universiti Sains Malaysia
University of Arizona
University of British Columbia
University of California, San Diego
University of Colorado
University of Florida
University of Illinois at Chicago
University of Maryland, Baltimore
University of Michigan
University of Minnesota
University of Mississippi
University of New Mexico
University of Rhode Island
University of Sheffield
University of Twente
Utrecht University
West Virginia University
Note Regarding Chrome and PDF Downloads:
Chrome has a known issue regarding automatic download of PDFs into a Chrome browser window (vs downloading into the Adobe Acrobat/PDF application). If the PDF is not correctly downloading when using a Chrome browser, you can download in Chrome by changing your settings or by using an alternate browser type (eg, Internet Explorer, Firefox, etc). Instructions for changing your Chrome settings for PDF download can be found here.
Subscribe to HEOR News and Events
Connect with the global community of healthcare stakeholders and stay informed on the latest and greatest happenings in the world of HEOR.

