Enhancing Access to Innovative Medical Technologies Through Early Value Assessment in the United Kingdom
Denise So, MSc, Evidera, part of PPD clinical research business, Thermo Fisher Scientific, Hammersmith, London, England, United Kingdom; Stacey Chang-Douglass, PhD, Pro Bono Health Economist Network, St Albans, Hertfordshire, England, United Kingdom; Naomi Stapleton, BSc, Evidera, part of PPD clinical research business, Thermo Fisher Scientific, Hammersmith, London, England, United Kingdom; Emily Hearne, MSc, Evidera, part of PPD clinical research business, Thermo Fisher Scientific, Hammersmith, London, England, United Kingdom

Overview of innovative MedTech in the United Kingdom
In February 2023, the UK Department of Health and Social Care published the government’s 5- to 10-year plan to support the use of innovative medical technologies (MedTech) to support patient care and clinical outcomes.1 Innovative MedTech include digital health technologies, such as therapies and systems that can improve patient health or increase healthcare system capacity.2,3 Examples of innovative MedTech include mobile treatment apps, wearable devices, telemedicine platforms, imaging systems, electronic health records, surgical instruments, and artificial intelligence–powered diagnostic tools.
Innovative MedTech have the potential to address several unmet needs in healthcare by improving the efficiency and capacity of healthcare systems, as well as by allowing patients to be diagnosed, receive treatment, and be monitored remotely. By providing patients with access to healthcare regardless of location, innovative technologies that deliver treatment could potentially reduce waiting lists, decrease the number of hospital visits, reduce costs associated with face-to-face healthcare consultations, and help to address inequality by providing a treatment option for patients who are unable or unwilling to travel for in-person appointments.3,4
The National Institute for Health and Care Excellence (NICE) is a nondepartmental public body sponsored by the Department of Health and Social Care in England. NICE publishes a wide range of guidance on how to improve health and social care, including appraisals on the clinical and cost-effectiveness of new health technologies.5 In June 2022, a new early value assessment (EVA) process was introduced to accelerate the assessment of innovative MedTech by NICE. The aims of EVA include:3
- Allowing patients and the National Health Service (NHS) in England to benefit from earlier access to promising innovations that address unmet needs;
- Facilitating adoption of new innovative MedTech and supporting evidence generation for new technologies; and
- Verifying that innovative MedTech deliver the expected benefits and ensuring these technologies provide value for money for the NHS.
This article provides an overview of the NICE EVA program, including published EVA guidance, evidence gaps highlighted during the assessments, and next steps for conditionally recommended technologies.
"By providing patients with access to healthcare regardless of location, innovative technologies that deliver treatment could potentially reduce waiting lists, decrease the number of hospital visits, and reduce costs associated with face-to-face healthcare consultations."
EVA for innovative MedTech in the United Kingdom
Innovative MedTech that address unmet need(s) within an NHS priority area, lack sufficient evidence for a full NICE appraisal, and are currently being used in the NHS or are planned for uptake within the next 6 months may be suitable for EVA.3,6 The initial priority topics identified for EVA were mental health, cardiovascular, early cancer detection, and technologies that boost healthcare capacity.6 During the EVA process, NICE assesses the available evidence for a single technology or a number of similar technologies and makes one of the following recommendations based on potential benefits and harms to patients, carers, and the system (including costs):3
- Conditionally recommended for use while further evidence is generated: For technologies that are likely to address an unmet need and where any risks or uncertainties can be sufficiently mitigated.
- Recommended in research only: For technologies where there is considerable uncertainty about whether an unmet need would be addressed or about whether potential benefits outweigh potential risks.
- Not recommended for use: For technologies that are not expected to address an unmet need and/or may be harmful even in a research context.
Published and ongoing EVAs
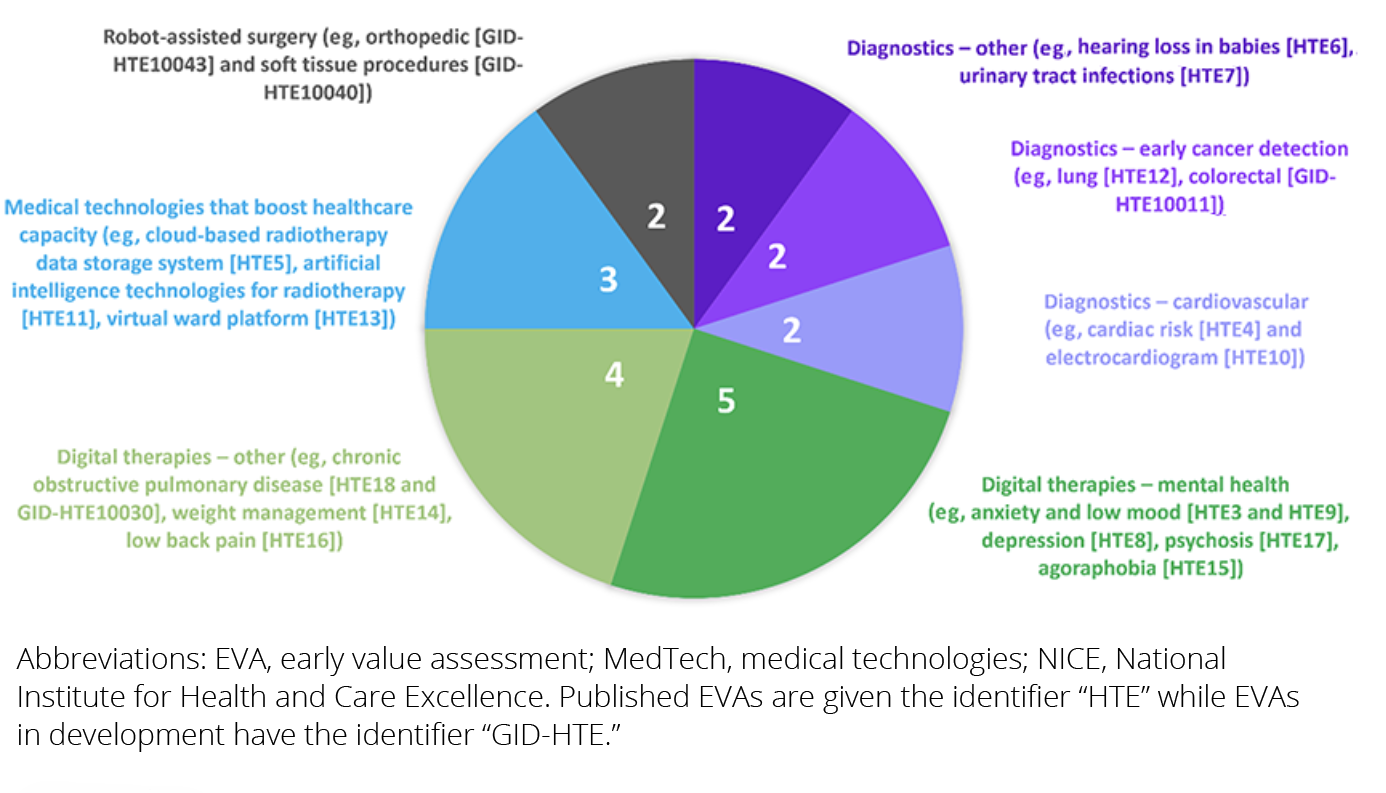
As of August 29, 2024, 16 EVA health technology evaluations (HTEs) have been published and 4 are currently in development. Each evaluation has a reference number on the NICE website, with the prefix “GID-HTE” applied while the evaluation is ongoing and the prefix “HTE” applied when the EVA guidance has been published. As summarized in the Figure, digitally enabled therapies to treat mental health disorders (anxiety and low mood [HTE3 and HTE9], depression [HTE8], agoraphobia [HTE15], and psychosis [HTE17]) are the most common EVA topics currently, followed by digitally enabled therapies for other conditions such as chronic obstructive pulmonary disease (HTE18 and GID-HTE10030), weight management (HTE14), and low back pain (HTE16).4,7
A total of 120 innovative MedTech have been included in the evaluation scope across the 16 published EVA guidance documents, of which 57 have been conditionally recommended for use while further evidence is generated.4 Recommended innovative MedTech were expected to address an unmet need within the NHS.4
- Diagnostic technologies may be quicker and more accessible than in-person diagnostics, reducing wait time for diagnosis and allowing patients to begin treatment or make lifestyle changes earlier.
- Digitally enabled therapies could provide an alternate or additional treatment option to existing therapies, reduce disease symptoms, improve patients’ ability to function in everyday life, reduce wait times for in-person treatment, and free up NHS resources to increase capacity elsewhere.
- Innovative technologies could boost healthcare capacity by improving knowledge sharing, increasing efficiency and consistency throughout the NHS, and reducing pressure on hospitals.
In some instances, patients may find the flexibility, comfort, and convenience of digitally enabled therapies preferable to in-person appointments. The EVA guidance for KardiaMobile 6L (AliveCor), a 6-lead, handheld electrocardiogram device that can be used during home visits by health professionals (HTE10), noted that patients preferred KardiaMobile 6L due to it being easier to use and less intrusive than standard electrocardiogram devices, which require patients to attend medical centers.8
Although early data for conditionally recommended innovative MedTech suggest these technologies could advance patient care and/or improve health and social care systems, many uncertainties are not currently addressed by available evidence. These can include safety concerns, patient uptake, patient adherence to digitally enabled therapies, set-up costs, and costs associated with training individuals (including NHS staff, patients, and caregivers) on how to use the technologies.4 These data gaps could be addressed by conducting real-world studies.
"Although early data for conditionally recommended innovative MedTech suggest these technologies could advance patient care and/or improve health and social care systems, many uncertainties are not currently addressed by available evidence."
Additionally, some technologies may pose equality issues. For example, the EVA guidance for technologies to manage nonspecific low back pain (HTE16) noted that digitally enabled therapies may be unsuitable for people who do not have the appropriate devices (eg, smartphones or tablets), have limited Internet access, or cannot read or understand health-related information (eg, individuals who do not speak English).9 Individuals who are unfamiliar with digital technologies, have visual impairments, or have problems with manual dexterity may have limited benefit from digitally enabled therapies.9
Figure. Topics across all published and ongoing NICE innovative MedTech EVAs as of August 29, 20244,7
From recommendation to clinical practice
There is currently limited clinical evidence on the effectiveness of innovative MedTech in the UK/NHS setting. As part of the EVA process, NICE develops evidence-generation plans for conditionally recommended innovative MedTech that highlight key evidence gaps and suggest methods to address them.3,6
In a draft proposal set out by NICE and NHS England in May 2024 introducing a new integrated pathway for introduction of medical technologies into the NHS, EVA is described as a “bridge” to the full NICE appraisal that identifies additional evidence needed for full appraisal, facilitates early access to promising new technologies, and enables the technology developer to collect the necessary evidence in the NHS setting.10 Moreover, NICE and NHS England expect EVA guidance to benefit clinicians and commissioners by allowing them to prepare for new MedTech that address patient need and clinical demand and are likely to be adopted into the NHS.10 Public engagement on the draft proposal ended on August 15, 2024, and a report will be published in the future addressing the issues raised during the consultation.10
"Early value assessment is described as a “bridge” to the full NICE appraisal that identifies additional evidence needed for full appraisal, facilitates early access to promising new technologies, and enables the technology developer to collect the necessary evidence in the NHS setting."
As of August 29, 2024, NICE has published 14 evidence-generation plans (1 for each published EVA except HTE4 and HTE7). The plans included recommendations on study designs, priority outcomes for data collection, possible data sources (such as general practitioner electronic records or existing databases), and relevant stakeholders (such as patient organizations and clinical experts who could help with data collection and analysis).3,4,6 Technology developers must contact NICE within 6 months of plan publication and annually thereafter during the period of evidence generation (2 to 4 years). Any substantial risks with evidence collection, new safety concerns, or significant changes to the technology must be reported to NICE as soon as possible. At the end of the evidence-generation period, the technology developer will submit its evidence to NICE for review and assessment of whether the innovative MedTech should be routinely adopted within the NHS.4
"Our understanding of how patients and the NHS can benefit from adoption of new innovative MedTech will continue to evolve over the next few years as additional studies are conducted to address evidence gaps and uncertainties flagged by NICE for the conditionally recommended technologies."
Conclusions
The number of EVA evaluations is expected to increase in the future as the innovative MedTech industry continues to develop and integrate into existing healthcare systems. Our understanding of how patients and the NHS can benefit from adoption of new innovative MedTech will continue to evolve over the next few years as additional studies are conducted to address evidence gaps and uncertainties flagged by NICE for the conditionally recommended technologies.
References
- The medical technology strategy: one year on. Department of Health & Social Care. https://www.gov.uk/government/publications/medical-technology-strategy-one-year-on/the-medical-technology-strategy-one-year-on. Published April 9, 2024. Accessed August 29, 2024.
- Medical technologies evaluation programme methods guide. National Institute for Health and Care Excellence. https://www.nice.org.uk/process/pmg33/chapter/introduction. Published August 21, 2017. Accessed August 29, 2024.
- Early value assessment interim statement. National Institute for Health and Care Excellence. https://www.nice.org.uk/process/pmg39/chapter/introduction. Published December 15, 2022. Accessed August 29, 2024.
- Guidance: early value assessment (EVA). National Institute for Health and Care Excellence. https://www.nice.org.uk/search. Search term: Early Value Assessment (EVA). Search date August 29, 2024.
- About. National Institute for Health and Care Excellence. https://www.nice.org.uk/about. Accessed August 29, 2024.
- Early value assessment (EVA) for medtech. National Institute for Health and Care Excellence. https://www.nice.org.uk/about/what-we-do/eva-for-medtech. Accessed August 29, 2024.
- Guidance: NICE advice and quality standards: in development. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/indevelopment. Search term: Early Value. Search date August 29, 2024.
- KardiaMobile 6L for measuring cardiac QT interval in adults having antipsychotic medication. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/hte10. Published September 26, 2023. Accessed August 29, 2024.
- Digital technologies for managing non-specific low back pain: early value assessment. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/hte16. Published March 5, 2024. Accessed August 29, 2024.
- Building an integrated, rules-based medical technology (medtech) pathway: engagement on proposals. NHS England. https://www.england.nhs.uk/long-read/building-an-integrated-rules-based-medical-technology-medtech-pathway-engagement-on-proposals/. Published May 22, 2024. Updated May 23, 2024. Accessed August 29, 2024.
Disclosures/Acknowledgements
Stacey Chang-Douglass serves as a member of NICE Medical Technologies Advisory Committee (MTAC); the views expressed in this article are those of the author and not necessarily those of NICE or NICE MTAC. Denise So, Naomi Stapleton, and Emily Hearne declare that they have no conflicts of interest.

