Are There Specificities for Assessing Quality of Life and Utilities in Rare Diseases for Economic Evaluation in France: A Case Study of Published CEESP (Health Economic and Public Health Committee) Opinions
Sarah Sadeuk-Benabbas, PharmD, Alira Health, Paris, France; Erwan Autin, Msc, Alira Health, Paris, France; Anne-Line Couillerot, Alira Health, Paris, France; Valérie Clément, PhD, Université de Montpellier, Montpellier, France

Background
As part of the responsibility of the French National Authority for Health (HAS) for assessing the cost-effectiveness and budget impact of healthcare products, the Commission for Economic and Public Health Evaluation (CEESP) issues an economic opinion that is sent to the Healthcare Products Pricing Committee (CEPS) to help guide price negotiations.
The economic analysis must follow the principles outlined in HAS’s economic evaluation guidance documents to ensure valid information. Based on HAS’s technical review of the manufacturer’s application, the CEESP may express concerns about the methodology used in the economic evaluation, such as efficacy, safety, utility score, and costs. A reservation is noted if a methodological choice does not comply with current recommendations. The severity of the reservation depends on the argument’s acceptability and the impact of the methodological choice on the results, especially regarding uncertainty.
Reservations are graded based on the product being assessed and the analysis context. For example, noncompliance in a quality-of-life evaluation method is more impactful if health-related quality of life is a significant outcome for the product or disease.
The reservation levels are categorized as follows: Minor reservations do not meet current recommendations but have minimal impact; important reservations, while justifiable, significantly affect findings; and major reservations invalidate the economic evaluation, rendering it unreliable despite any justifications.
Introduction
Measuring quality of life (QoL) in rare diseases can be challenging as in small samples or populations without cognitive ability to answer QoL questionnaires requires a proxy (third person) (including pediatric).1 These challenges are identified in the French Health Technology Assessment (HTA) guidelines for health economics, but no standards are proposed.2 A recent review of international HTA processes for assessing orphan drugs showed that there are generally no specific processes or requirements for these drugs, rather adjustments to the usual standards.3
Despite the lack of dedicated methodological guidelines and procedures, development of orphan drugs presents specificities and challenges beyond QoL measurements, potentially adding a level of complexity for HTA and decision making.4
"A recent review of international HTA processes for assessing orphan drugs showed that there are generally no specific processes or requirements for these drugs, rather adjustments to the usual standards."
One of the main challenges is the lack of long-term efficacy data—which is generally accepted for orphan drugs, resulting in the use of surrogate endpoints5—especially for mortality, making it harder for the health economic evaluation to conduct a proper cost-effectiveness analysis, incorporating uncertainty around life-years estimates. It raises the need for robust QoL and utility measures to conduct cost-utility analyses that could be used for decision making.
Another specificity of orphan drugs is pricing and economic model for tariffication; addressing small prevalent diseases, research and development (R&D) costs could generally not be borne through large sales, leading to high treatment costs at the patient level. At the HTA level for health economics, it usually leads to high incremental cost-effectiveness ratios (ICERs).6 As a result, some countries attempted to adapt their decision-making framework as in the United Kingdom (National Institute for Health and Care Excellence [NICE]) with a dedicated ICER threshold for orphan drugs.7 However, such decision criteria do not exist in France, where health-economic assessment is only part of the pricing-setting procedure.a
This context raises 2 main questions in France:
- How is utility estimated and assessed in health economics for rare disease evaluations?
- How are resulting ICERs appreciated by CEESP in the absence of a specific framework?
Objectives
This study aims to assess the impact of the methodology used to estimate utility values on the conclusions of the CEESP for orphan drugs. This assessment will identify accepted deviations from guidelines for utility estimates in the context of rare diseases, presenting methodological challenges and sparse data.
Additionally, the study analyzes the conclusions of the economic assessment, with a focus on ICER levels, when validated by CEESP. The goal of this study is to identify from past decisions of CEESP if there is a de facto framework for economic assessment in the context of rare disease in France.
Methodology
The analysis is based on a review of CEESP opinions in rare disease (orphan drugs), published from 2014 to the end of 2021. A focus was done on QoL measures used by manufacturers and assessments by CEESP, including ICER level.
Relevant information for the research questions were reported in an analytical grid:
- General and administrative elements: product, date of assessment, indication, pediatric population or not;
- Claims of the manufacturer for HTA procedure: added value claimed (ASMRb,c) and obtained from French clinical HTA committee TC (transparency committee), target population, budget impact, technical exchange (written questions and answers) conducted or not during health economic assessment, hearing/observations from manufacturer on draft opinion;
- Conclusions’ synthesis: maximum grading of methodological reservationd and details (number and topic), ICER level, ICER qualification, committee’s conclusion, complementary data asked on utility;
- Economic evaluation’s details: data sources for utility, QoL questionnaire, patients’ capacity to answer questionnaires, person answering QoL questionnaire (patient, parent, other caregiver), integration of caregiver QoL, details of reservations on utility assessment.
Descriptive and quantitative analyses were performed to understand type of methodology accepted by CEESP for measuring health utility in rare disease contexts.
Results
Twenty-seven CEESP opinions on rare diseases were analyzed: the size of the target population varied from 75 to 8830 patients. Fourteen opinions included both pediatric and adult populations and one only pediatric.
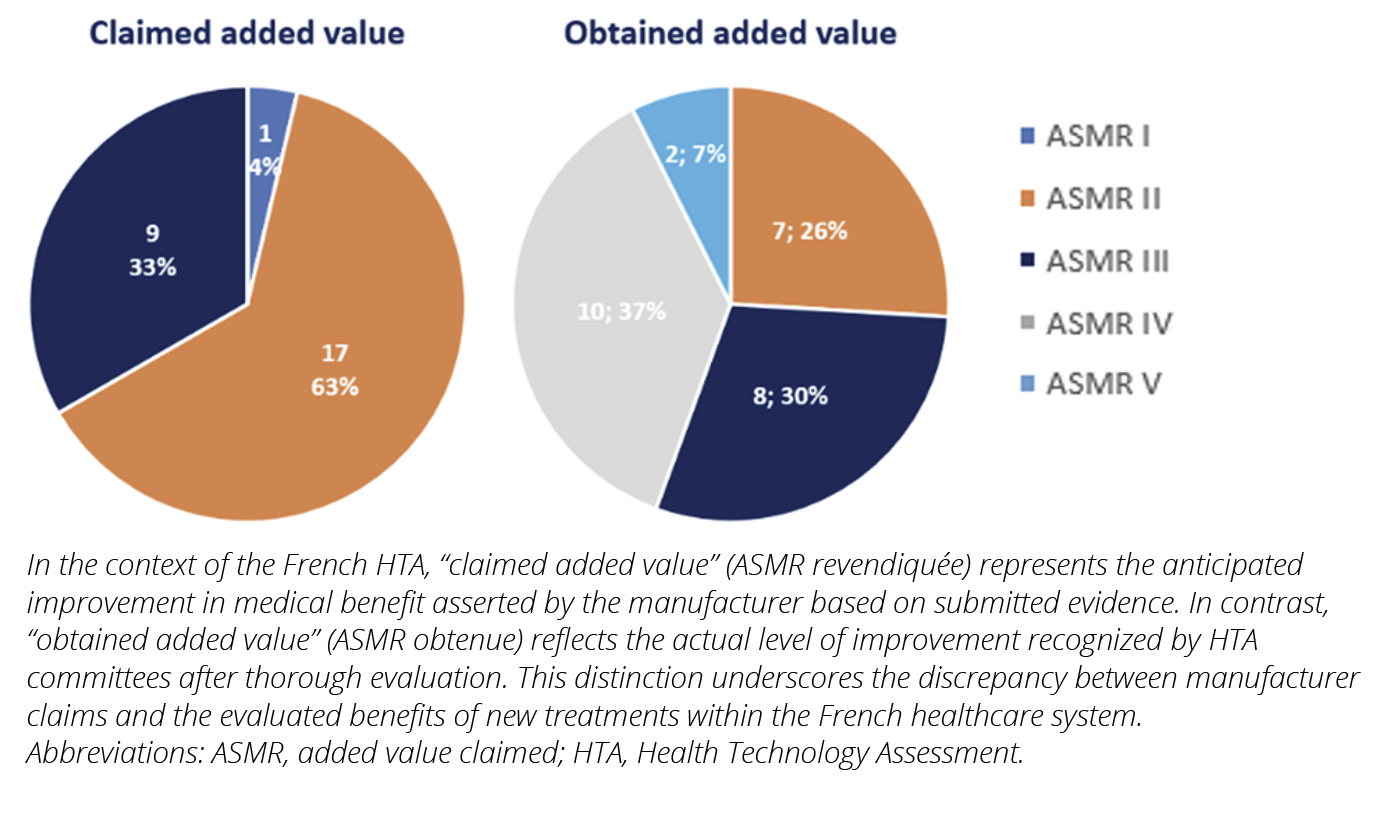
Claimed “added value” were mainly important and moderate, but obtained added values were lower (Figure 1).
As a comparison, in 2021, for all drugs assessed, excluding rare diseases, TC granted 0.8% major added value, 0.8% important, 22.7% moderate, 28.9% minor, and 46.9% inexistent.8 Proportionally higher “added value” for drugs assessed in this study could be explained by: i) demonstration of the eligibility to health-economic assessment being required for innovative drugs2; ii) context of rare diseases, with an important part of drugs claiming a change in care paradigm as few therapeutic options exists.
Across the economic assessments of the 27 drugs studied, 17 (63%) had important methodological reservations over at least one aspect of the economic evaluation, while 10 (37%) had at least one major reservation.
Figure 1. Claimed vs obtained added value
Challenges in utility assessment for orphan drugs
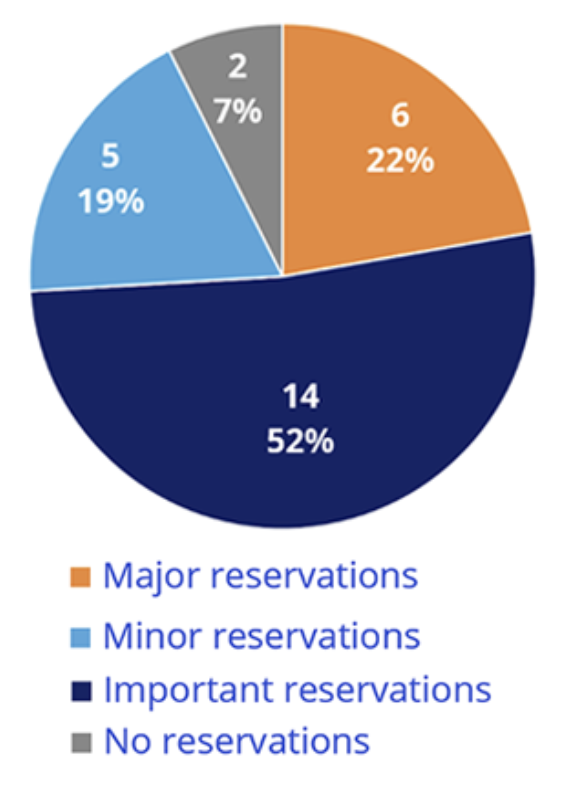
Out of the 27 opinions, only 2 (7%) had no reservation on estimation and implementation of health utility in the economic evaluation, and 52% (n=14) had at least one important reservation, denoting a significant estimated impact on the results of the economic evaluation.9
More strikingly, among rejected opinions (n=10), 55% (n=6) were due to inappropriate methods used to measure utilities, noting the importance of adequate QoL assessment (Figure 2).
Figure 2. Maximum methodological reserve level associated with utility
These results show the difficulties in obtaining and implementing robust utility estimates in economic evaluation in the context of rare disease, despite the fact that quality of life is an important component of these diseases.
Methodology rejected: role of data sources
Methodology recommended by HASe (Guideline 15): “The utility scores used to weight life-years should be estimated using a multi-attribute approach, comprising the collection of health state data from patients via a generic questionnaire and the valuation of health states according to the preferences of the general population.”2
In 80.7% of opinions studied, patients answered their own QoL through the EQ-5D questionnaire, as recommended by HAS. No differences were noted between adult and pediatric populations and no proxy respondent had to be asked when the data came from a clinical trial. Except for 2 opinions, patients had the cognitive capacity to respond directly to QoL questionnaires.
Caregiver utility was not considered in the opinions studied, except for one including it in a sensitivity analysis. Despite being potentially important in a rare disease context (especially for pediatric populations), this could be explained by poor data availability (or quality) even if it enters in the scope of HAS’s guidelines.
In one case, a cost-efficacy analysis was proposed in anticipation of methodological difficulties associated with utility estimates.
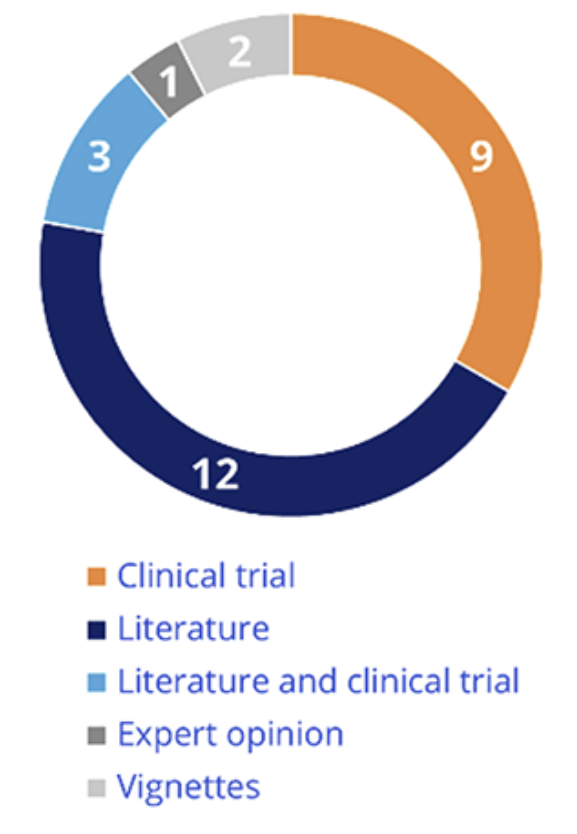
Repartition of data sources for utility is presented in Figure 3. Health utility was informed from literature in most cases, clinical trials, or both.
Figure 3. Source of health-related quality of life data
For 5 opinions with major reservation on utilities (out of 6), CEESP rejected the methodology considering inappropriate data source:
- Vignette study (n=2), rejected because they were not completed by patients themselves
- Expert opinion (n=1), HRQoL was not estimated by patients as well
- Disease-specific questionnaires (n=2)
For the opinions without major reservation, when the methodology was considered appropriate by CEESP, it was supported by robust data sources: literature (n=11), clinical trials (n=7), or both (n=3).
These results show no specificity in the context of rare disease and that the general method recommended by HAS is applicable.
Methodology accepted: challenge of willingness-to-pay for orphan drugs
When the methodology was accepted, CEESP assessed efficiency of the drugs, but issues appeared with higher ICER levels. For 19 opinions (70%) with efficiency conclusions, the average ICER was 827,000 €/QALY (ranges from 70,651 €/QALY to 2,700,000 €/QALY) (Figure 4).
Figure 4. ICER levels and qualification by CEESP
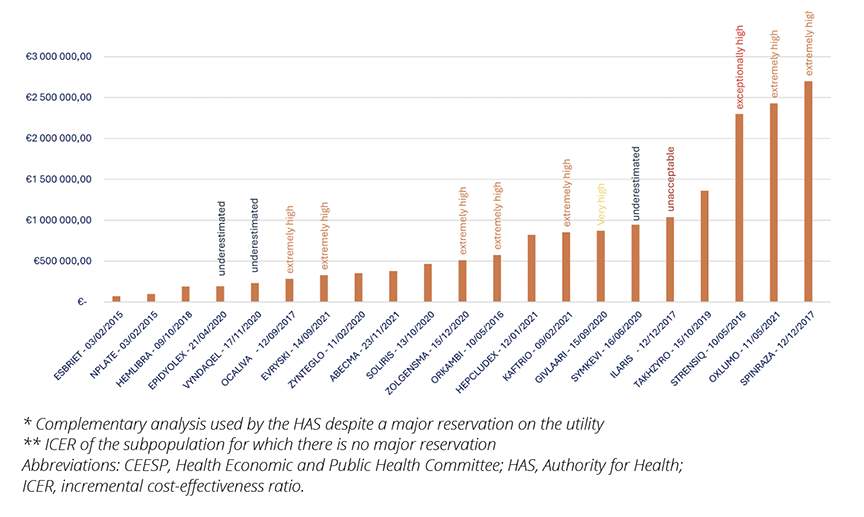
In 50% of the cases, CEESP considered these ICER levels to be extremely high and in 2 cases, they were qualified as “exceptionally high” or even “unacceptable.” By way of comparison, the average ICER for all innovative medicines subjected to economic evaluation by the CEESP between 2014 and 2020 was 287,821 €/QALY, and only 25% of ICERs were higher than 239,145 €/QALY.10
The ICER levels in rare disease are well above traditional value for money thresholds, emphasizing the specific problem of high-cost orphan drug pricing: how to articulate cost efficiency, affordability, and social value in the assessment of healthcare value.11
Implications for HTA Practitioners
- Prioritize the use of EQ-5D data or employ mapping techniques from clinical trials whenever feasible, particularly in the context of orphan drugs where robust QoL data can significantly impact HTA outcomes.
- Consider the utilization of utility values from published literature, including proxies for disease, similar health states or events modeled. Ensure these values are contextually appropriate for rare diseases.
- Encourage the development and adoption of advanced methodologies for capturing QoL impacts in rare disease populations, such as patient-reported outcomes (PROs) tailored to the specific challenges of orphan drug evaluation.
Conclusion
Despite methodological difficulties associated with utility assessment in rare diseases, most of the analyses studied implemented CEESP guidelines without specific issues related to HRQoL measures, demonstrating that CEESP general guidelines can be effectively applied in the context of orphan diseases.
Beyond the methodology, when results can be estimated, they illustrate the debate in academic literature questioning the relevance of higher thresholds for rare diseases. In this context, other criteria may be considered by decision-makers as equity to treatment access, supporting research and development in the field of rare diseases through higher prices, or budget impact considerations.
References
- Prosser LA, Hammitt JK, Keren R. Measuring health preferences for use in cost-utility and cost-benefit analyses of interventions in children: theoretical and methodological considerations. Pharmacoeconomics. 2007;25(9):713‑726.
- Choices in methods for economic evaluation. Haute Autorité de Santé. https://www.has-sante.fr/jcms/r_1499251/en/choices-in-methods-for-economic-evaluation. Published July 29, 2020. Updated November 6, 2020. Accessed November 11, 2024.
- Stafinski T, Glennie J, Young A, Menon D. HTA decision-making for drugs for rare diseases: comparison of processes across countries. Orphanet J Rare Dis. 2022;17(1):258.
- Nicod E, Annemans L, Bucsics A, Lee A, Upadhyaya S, Facey K. HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy. 2019;123(2):140‑151.
- Doctrine de la commission de la transparence (CT): Principes d’évaluation de la CT relatifs aux medicaments en vue de leur accès au remboursement. Haute Autorité de Santé. https://www.has-sante.fr/upload/docs/application/pdf/2021-03/doctrine_ct.pdf. Published February 15, 2023. Accessed November 11, 2024.
- Winquist E, Coyle D, Clarke JTR, et al. Application of a policy framework for the public funding of drugs for rare diseases. J Gen Intern Med. 2014;29(S3):774‑779.
- Highly specialised technologies guidance. National Institute for Health and Care Excellence. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-highly-specialised-technologies-guidance. Accessed November 11, 2024.
- Rapport d’activite 2021 de la Comission de la transpatence. Haute Autorité de Santé. https://www.has-sante.fr/upload/docs/application/pdf/2022-06/rapport_dactivite_2021_de_la_ct_2022-06-29_22-01-10_795.pdf. Published June 29, 2022. Accessed November 11, 2024.
- Doctrine of the Commission for Economic and Public Health Evaluation: CEESP evaluation principles for healthcare products for pricing purposes. Haute Autorité de Santé. https://www.has-sante.fr/upload/docs/application/pdf/2021-09/doctrine_de_la_ceesp_version_anglaise_2021-09-29_11-14-2_803.pdf. Published July 6, 2021. Accessed November 11, 2024.
- Kergall P, Autin E, Guillon M, Clément V. Coverage and pricing recommendations of the French National Health Authority for innovative drugs: a retrospective analysis from 2014 to 2020. Value Health. 2021;24(12):1784-1791.
- Danzon P. Affordability challenges to value-based pricing: mass diseases, orphan diseases, and cures. Value Health. 2018;21(3):252-257. doi: 10.1016/j.jval.2017.12.018.
a Health-economic assessment is produced by CEESP (Health Economic and Public Health Committee) and then economic information assessed is used by the decision makers: pricing committee for healthcare products (CEPS), as an input for price negotiations.
b In France, ASMR levels (Amélioration du Service Médical Rendu) indicate the degree of improvement a new drug or medical device offers compared to existing treatments, ranging from ASMR I (major improvement) to ASMR V (no improvement). These levels help determine reimbursement and pricing within the healthcare system.
c Economic assessment in France concerns only drugs with claimed ASMR ≥ III (Major, level I, important level II, and moderate level III, on a scale up to IV minor and V absence of added value).
d Methodological reservations from CEESP are rated from major (invalidation of the analysis), important (strong uncertainty and impact), to minor (deviation justified or low impact expected).
e HAS stands for the “Haute Autorité de Santé,” which translates to the High Authority of Health. The HAS is an independent public authority in France responsible for assessing health products, treatments, and medical practices to ensure quality, safety, and effectiveness in healthcare.

