Implementing a Direct-to-Patient Application to Recruit Diverse Patients and Collect Real-World Data: A Case Study
Jackie Vanderpuye-Orgle, PhD, Parexel, Los Angeles, CA, USA; Sumana Ramayanam, MBA, Parexel, Morrisville, NC, USA; David Barratt, BSc, Parexel, Uxbridge, UK; Winston W. H. Eng, MS, Parexel, Seattle, WA, USA; Luke Thayer, BS, Parexel, Boston, MA, USA
Introduction
Factors such as digital literacy, cultural differences, privacy concerns, data quality, systemic biases, and access to healthcare can affect the enrollment and participation of diverse racial and ethnic minorities, genders, and older adults in clinical research. Marginal sociodemographic groups are often underrepresented or excluded from clinical trials, limiting the generalizability and applicability of the findings to real-world settings and populations, as emphasized by the US Food and Drug Administration (FDA) guidance on enhancing diversity in clinical trials.1
The COVID-19 pandemic also highlighted the need for innovative and patient-centric approaches for decentralized and remote clinical trial conduct, while ensuring data integrity and patient engagement.2 The FDA also noted efforts to make trial participation less burdensome for participants, including the use of online/social media recruitment strategies.
Parexel implemented a direct-to-patient application that leveraged digital and data innovation to recruit patients and collect real-world data (RWD) for a study on oral anticoagulant prophylaxis among patients with cardiovascular diseases. The study, named Resolve, utilized social media to reach out to potential participants across the United States, collected their consent and survey data through a secure platform, and applied a novel tokenization technique to link their data with commercially available claims data. The study aimed to demonstrate the feasibility and value of using a direct-to-patient application to enhance participant engagement, diversity, privacy, real-world data linkage and insights in clinical trials.
Methods
The Resolve study was conducted between May 2021 and July 2021 and involved multiple technology and data partnerships to ensure a seamless patient experience and data flow. The study protocol and documents were reviewed and approved by an independent institutional review board. The study population consisted of patients aged 40 years and older with cardiovascular diseases who were recruited through social media platforms. The recruitment strategy was designed to target a specific group of patients who are being treated with anticoagulants.
"Marginal sociodemographic groups are often under-represented or excluded from clinical trials, limiting the generalizability and applicability of the findings to real-world settings and populations."
The interested patients were directed to a landing page that provided information about the study and the eligibility criteria. The eligible patients were then invited to complete an online informed consent form and a survey questionnaire through a third party’s platform. The survey questionnaire collected self-reported data on the patients’ demographics, health status, medication use, comorbidities, mental health, and satisfaction with their treatment and care. The survey also asked the patients for their willingness to provide additional consent for tokenization and data linkage. Tokenization is a process of generating unique identifiers (tokens) based on the patients’ personally identifiable information, such as name, date of birth, gender, and state. The tokens enable data linkage and facilitate the assessment of patient engagement, recruitment, and feasibility while protecting the patients’ privacy and identity.3 The first 100 patients who consented to tokenization and provided their personally identifiable information were compensated with a $50 gift card. The tokens were generated using the patients’ identification data and were used to match and link with the patients’ data in commercially available claims databases. The claims data provided longitudinal information on the patients’ diagnoses, procedures, prescriptions, and healthcare resource utilization. The data linkage enabled a comprehensive evaluation of the patients’ journeys, outcomes, and treatment patterns.
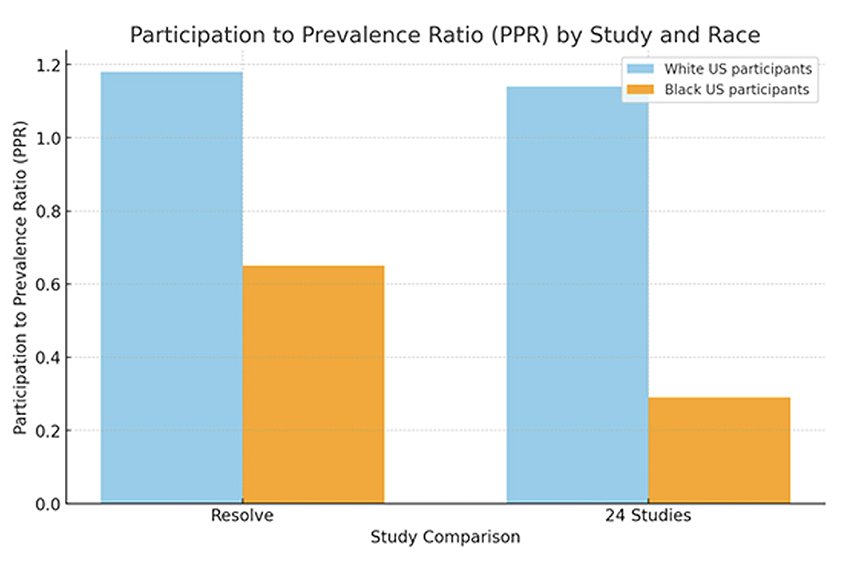
Participation of Black US residents in the Resolve study was compared to clinical trials of 24 cardiovascular drugs granted FDA approval (2006-2020).4 The patient participation to prevalence ratio (PPR) is determined by dividing the percentage of a specific group among trial participants by the percentage of the same group within the disease population.
The data analysis was performed using descriptive statistics, graphical methods, and comparative tests. The treatment pattern of oral anticoagulant medication was analyzed and visualized using an Alluvial plot/Sankey diagram method.
Results
A total of 676 patients in the United States participated in the survey, of which 643 (95%) consented to tokenization and 334 provided their personally identifiable information. Tokens were generated successfully for 305 patients, and 265 patients were linked to tokens in the claims data (Figure 1). The demographic breakdown of the participants was as follows: 51% females and 49% males; median age: 66 years (42-85); 85.7% Caucasian; 6.4% Black; 1.5% Latino;
1% Asian American; and 4.5% mixed race. The median annual income among the participants was $27,500 ($2500 to $162,500).
Figure 1. Patient funnel demonstrates the study’s success in recruiting specific patients while protecting patients’ privacy
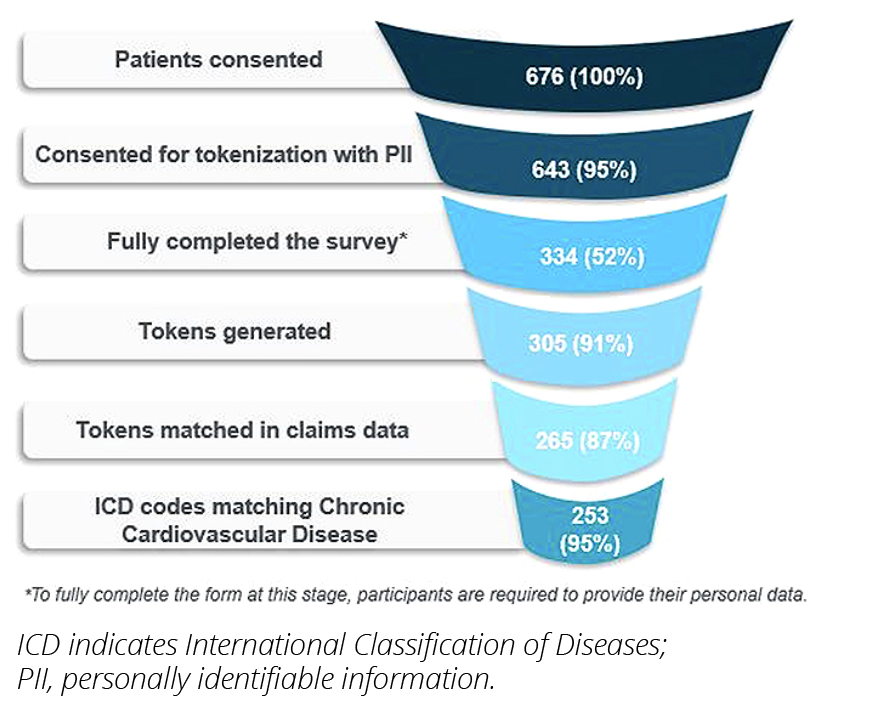
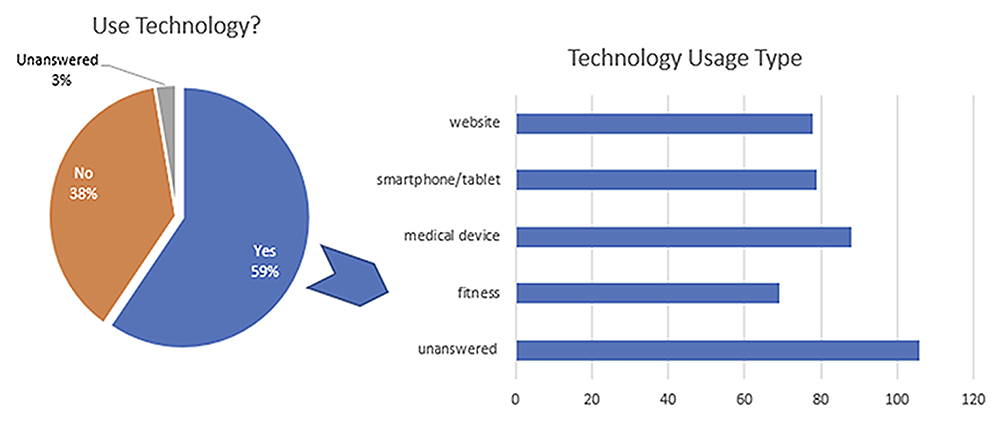
The PPR for Black participants in the Resolve study was 0.65, which was much higher than the PPR of 0.29 in the 24 cardiovascular studies which were the basis of approval for drugs.4 (Figure 2). The Resolve study also exhibited a strong representation of participants of mixed races, Latinos, and Native Americans, as well as women and older adults, compared to previous studies and the overall patients on oral anticoagulants in a commercially available claims database. Throughout the whole study, patients had full control of their data privacy. The study also provided valuable insights into the patients’ perceptions, preferences, and experiences with their treatment and care. The survey data revealed that 59.6% of the participants used technology for health and fitness or to monitor health issues, such as smartwatches, fitness trackers, etc. The most common type of technology used was a medical device (33.2%), followed by smartphone/tablet (29.8%), website (29.4%), and fitness tracker (26.0%) (participants could select multiple choices) (Figure 3). Most participants (85.5%) reported that they would be interested in participating in future clinical trials.
Figure 2. Improved sample diversity in Resolve study relative to existing literature.
The claims data enabled a more objective and comprehensive assessment of the patients’ clinical outcomes, death records, and comorbidities. The claims data showed that the most common comorbidities among the participants were hypertension (57.0%), diabetes (36.1%), and hyperlipidemia (29.7%), and other symptoms included shortness of breath (41.8%).
Figure 3. Technology use among the Resolve study population.
It is important to note that survey data alone do not cover the entire spectrum of diseases and are limited to a predefined set of options. Additionally, the self-reported data represent a specific point in time. In contrast, claims data provide a longitudinal perspective, capturing patient history spanning 9 years before and after the study.
This Sankey diagram visualizes the sequential treatment pattern of oral anticoagulant medications. Each section of a column represents a different medication used, and the lines depict the progression of patient treatment over time (Figure 4). The diverse patient sample included in the study seemed to indicate dynamic treatment patterns across the respective options for blood thinners. These findings suggest the need for further research.
Conclusions
The Resolve study demonstrates the feasibility and value of using a direct-to-patient application to recruit diverse patients and collect RWD for clinical trials. The study leveraged social media, digital platforms, and tokenization techniques to reach out to hard-to-reach patient groups, collect their consent and survey data, and link their data with claims data, while ensuring their privacy and identity protection. The study achieved a good racial, gender, and older adult representation compared to prior clinical trials4-6; however, additional approaches may be needed to comprehensively include under-represented groups. It provided rich and comprehensive insights into the patients’ journeys, outcomes, and treatment patterns.7 Claims data were consistent with the targeted patient population for the Resolve study, indicating that the majority of the cohort experienced cardiovascular disease issues as well as breathing problems. This aligns with the study period coinciding with the COVID-19 pandemic, during which such health problems were more prevalent. Moreover, the data also revealed mental health issues among the older adult population, which were exacerbated during the pandemic. Furthermore, 3 patients passed away within a year after the survey was conducted: 1 due to suicide and 2 due to COVID-19.
"The study underscores the importance of enhancing patient diversity, engaging communities, and employing robust data-linking techniques to optimize interventions and improve patient outcomes."
Although the Resolve study showed a higher PPR rate for Black patients compared to previous trials, it is essential to acknowledge that this still indicates underrepresentation. Adequate representation is typically indicated by PPRs between 0.8 and 1.2, whereas underrepresentation is represented by a PPR below 0.8 and overrepresentation by a PPR above 1.2. Additionally, the PPR for White participants remains relatively high. In future studies, it will be crucial to engage with minority communities to capture a more diverse population. Language assistance can also play a role in facilitating inclusivity and participation.
The study also revealed the patients’ high interest and willingness to participate in future clinical trials, especially if they were offered incentives, convenience, and access to new treatments. The detailed race information collected, including the mixed race, was in line with the FDA’s new guidance on collecting race and ethnicity data in clinical trials and clinical studies.8 Finally, the study underscores the importance of enhancing patient diversity, engaging communities, and employing robust data-linking techniques to optimize interventions and improve patient outcomes.
References:
1. FDA. FDA Takes Important Steps to Increase Racial and Ethnic Diversity in Clinical Trials. [Press release]. FDA; 2022. Accessed March 14, 2024. https://www.fda.gov/news-events/press-announcements/fda-takes-important-steps-increase-racial-and-ethnic-diversity-clinical-trials
2. Golinelli D, Boetto E, Carullo G, Nuzzolese AG, Landini MP, Fantini MP. Adoption of digital technologies in health care during the COVID-19 pandemic: systematic review of early scientific literature. J Med Internet Res. 2020;22(11): e22280. doi:10.2196/22280
3. Clinical Leader. What is clinical trial tokenization? Clinical Leader. 2024. Accessed March 14, 2024. https://www.clinicalleader.com/doc/what-is-clinical-trial-tokenization-0001
4. Chen S, Ando H, Oren E, Yang J, Oh SS. Participation of Black US residents in clinical trials of 24 cardiovascular drugs granted FDA approval, 2006-2020. JAMA Netw Open. 2021;4(3): e212640. doi:10.1001/jamanetworkopen.2021.2640
5. Cacciatore S, Sibilio S, Salvatore M, Sasso FC. Management of coronary artery disease in older adults: recent advances and gaps in evidence. J Clin Med. 2023;12(16):5233. doi: 10.3390/jcm12165233
6. Vitale C, Fini M, Speziale G, et al. Representation of elderly and women in clinical trials. Int J Cardiol. 2017; 232:216-221. doi: 10.1016/j.ijcard.2017.01.018
7. Lee MY, Han S, Bang OY, et al. Drug utilization pattern of oral anticoagulants in patients with atrial fibrillation: a nationwide population-based study in Korea. Adv Ther. 2022;39(7):3112-3130. doi: 10.1007/s12325-022-02151-z
8. FDA. Collection of Race and Ethnicity Data in Clinical Trials and Clinical Studies for FDA-Regulated Medical Products. [Guidance document]. FDA; 2024. Accessed March 14, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials-and-clinical-studies-fda-regulated-medical

