How Can Manufacturers of Orphan Drugs for Rare Diseases Optimize Clinical Trial Design for Market Access Across the United States, France, Germany, and the United Kingdom?
Anne Runyan, BA, Envision Pharma Group, San Diego, CA, USA; Kruthika Murali Dhar, MS, MBA, Envision Pharma Group, Allen, TX, USA; Joe Honcz, BS Pharm, MBA, Envision Pharma Group, New Haven, CT, USA; Sheli Goldstein, BS, Envision Pharma Group, San Diego, CA, USA
Implications
Payer preferences for clinical trial design for orphan drugs for rare diseases differ across the United States, France, Germany, and the United Kingdom. Gaining a better understanding of these preferences can help manufacturers to best design clinical trials in order to achieve favorable market access in each country.
Introduction
Rare diseases impact around 60 million people in the United States and Europe. More than 6000 rare diseases have been identified; most have no cure, and often, no drugs with demonstrated efficacy.1
Orphan drugs for rare diseases offer significant clinical benefits in high unmet-need populations; therefore, payers have historically provided more flexibility in reviewing these drugs for market access decisions compared to conventional drugs. Furthermore, health technology assessment (HTA) bodies are willing to make exceptions to traditional cost-effectiveness analyses when reimbursing orphan drugs for rare diseases; therefore, these drugs often launch at a higher price point compared to conventional drugs. Notably, orphan drug prices are closer between the United States and Europe, compared to conventional drug prices. For example, a 2021 study found that conventional prescription drug prices are, on average, 2.56 times higher in the United States compared with other nations.2 However, a 2022 comparison of orphan drug prices in the United States versus Europe found that the average price ratio is 1.64.3
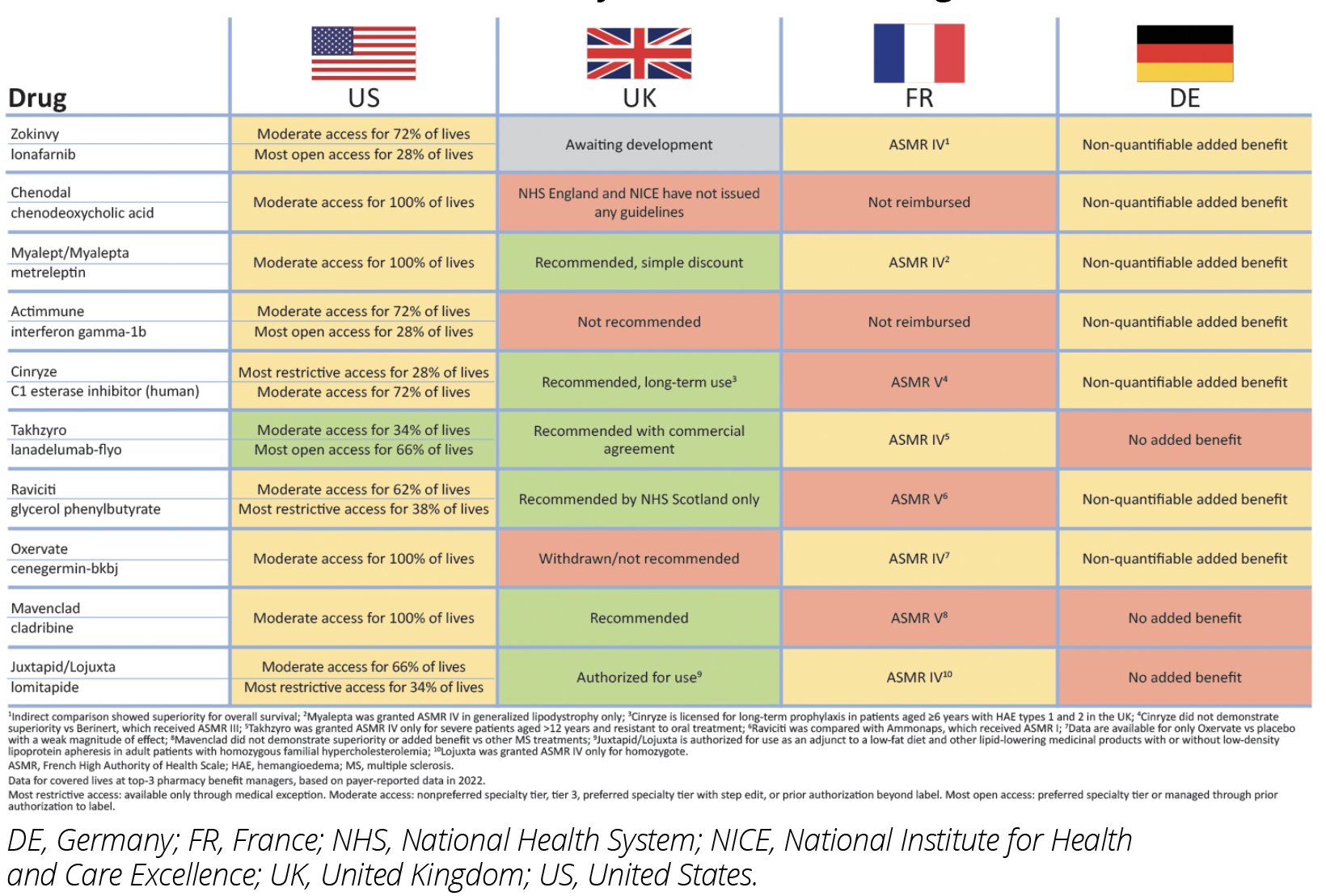
An analysis of the top 10 self-administered pharmacy benefit drugs by US Wholesale Acquisition Cost as of year-end 2021: Zokinvy (ionafarnib), Chenodal (chenodial), Myalept (metreleptin), Actimmune (interferon gamma-1b), Cinryze (C1 esterase inhibitor), Takhzyro (lanadelumab-flyo), Ravicti (glycerol phenylbutyrate), Oxervate (cenegermin-bkbj), Mavenclad (cladribine), and Juxtapid (lomitapide) found that 9 have moderate access in the United States, while Takhzyro has the most open access in the United States. In France, 5 have an ASMR IV while 3 have ASMR V.
In Germany, 7 have a nonquantifiable added benefit, while 3 have no added benefit. In the United Kingdom, 6 are recommended, while 1 has not yet been assessed, and 3 face restricted access.4-6 (Figure 1).
This research explores how data requirements and clinical trial design for orphan drugs for rare diseases differ between the United States and Europe and provides considerations for manufacturers regarding how to design clinical trials for orphan drugs for rare diseases to maximize access and reimbursement across geographies.
Figure 1: Market Access Decisions for Orphan Drugs for Rare Disease Therapies in the United States, France, Germany, and the United Kingdom
Research Methodology
Primary research was conducted with payers in the United States, France, Germany, and the United Kingdom to understand payers’ clinical trial design and data preferences for orphan drugs for rare diseases. In the United States, an online survey was developed and programmed in Qualtrics. It contained 53 questions and was sent through an email link to 29 payers, of which 24 responded. Payers could not advance without answering all questions and only completed surveys were recorded. Responses were collected from February to March of 2022. One qualitative in-depth telephone interview with one payer representing each of the countries: United States, France, Germany and the United Kingdom was completed in September of 2022. Payers were asked about their preferences for trial duration, sample size, placebo versus active comparator, and the impact of open-label extension data on their decisions for orphan drugs for rare diseases.
Results
A total of 24 US payers representing 116 million lives completed the quantitative survey, including 4 national managed care organization (MCO) representatives, 14 regional MCO representatives, 5 pharmacy benefit manager (PBM) representatives, and 1 state Medicaid payer. One US PBM Pharmacy Director, one government health insurance company advisor in Germany, 1 ex-Transparency Committee member in France, and 1 ex-member of National Institute for Health and Care Excellence (NICE) completed in-depth telephone interviews.
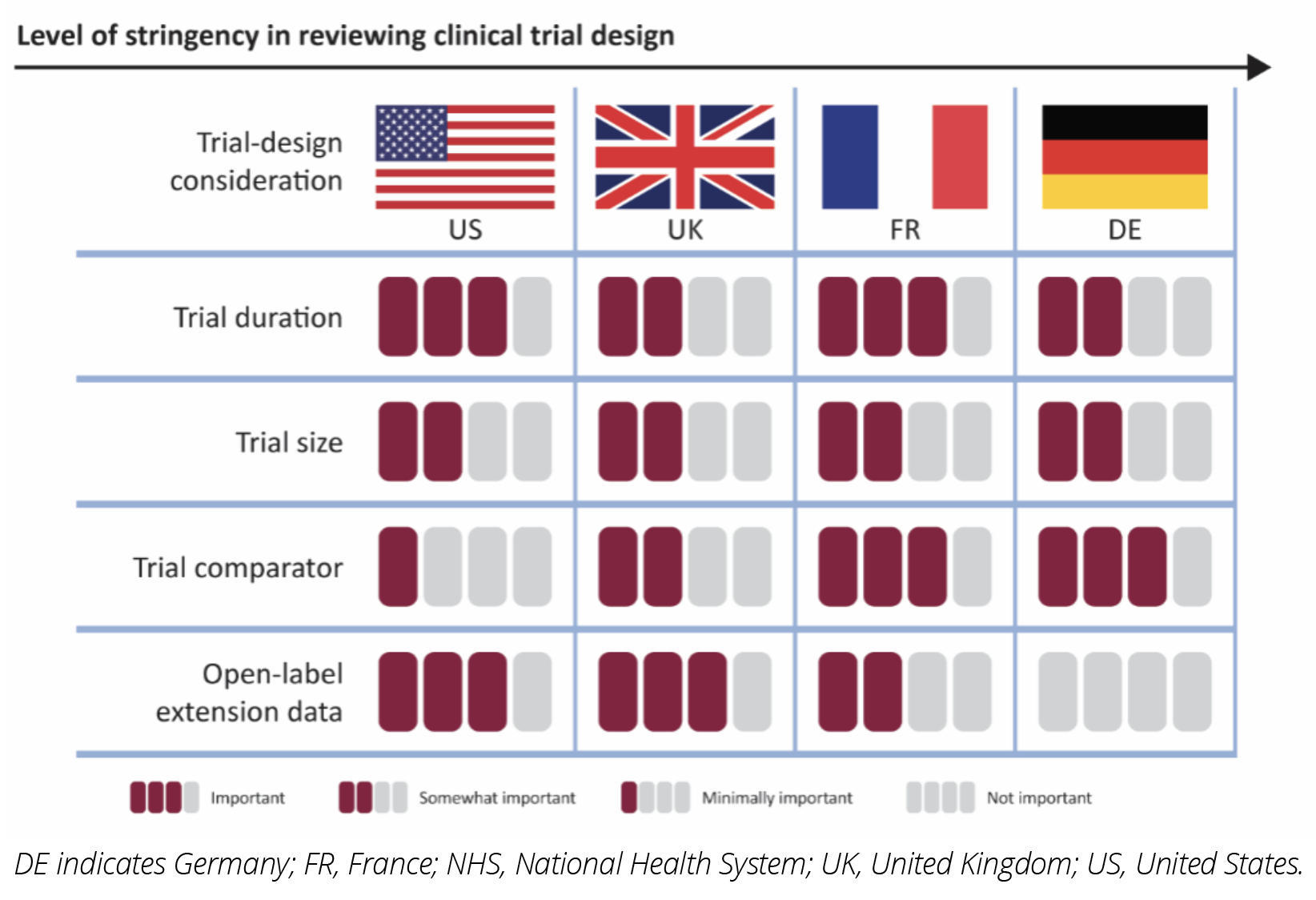
The importance of clinical trial design elements to payers across the 4 markets, in terms of their influence on the reimbursement decision, is summarized in Figure 2. Criteria that are categorized as “Not important” are not considered at all during decision making. For example, in Germany, open-label extension data are not important and are considered biased. “Minimally important” criteria are unlikely to be considered. For example, US payers place minimal importance on clinical trial comparators, as they routinely reimburse products that have been studied in placebo-controlled trials. “Somewhat important” criteria may be considered but are unlikely to influence the decision, whereas “Important criteria” are considered and will impact the decision. For example, open-label extension data could positively impact the formulary placement of a drug in the United States.
Figure 2: The Importance of Clinical Trial Design Elements to Payers Across the United States, France, Germany, and the United Kingdom
Trial Duration
In the United States, 20/24 payers indicated they are looking for trials of at least 12 months while 4 payers find trial durations between 6 and 12 months to be appropriate. In France, the payer stated that although the Transparency Committee prefers trials at least 2 years long, trials 6 months or shorter may be acceptable, especially if they involve diseases with rapid progression. They also noted that manufacturers can submit interim data for an initial coverage decision and provide additional data as they become available. In Germany, orphan drugs do not undergo a full review until they reach an annual sales threshold of €30 million. Of the 107 orphan substances reviewed by the Federal Joint Committee between January 2011 and June 2022, 20 exceeded the threshold (which was €50 million at the time).7 It is expected that more drugs will exceed the new, lower threshold. For drugs that exceed the threshold, trial duration is a formal criterion considered during the review, and payers prefer a duration of at least 6 months. In the United Kingdom, trial duration is a formal criterion in the NICE review, and payers require trials to be at least 6 months.
Trial Size
The sample size of the trial is not a formal criterion for US pharmacy and therapeutics (P&T) committee review. Of the 24 payers, 21 indicated they are moderately concerned with smaller sample sizes for orphan drugs and for rare disease therapies, 2 indicated they are not at all concerned, and 1 indicated they are significantly concerned. The French respondent had little concern with smaller trials, noting they are more concerned about the magnitude of the benefit observed. They cited the example of spinal muscular atrophy, where the trial included 12 patients per treatment arm, and all 12 responded, resulting in a decision that the drug offered an important improvement relative to the current standard of care. In Germany, trial size is not a formal criterion, but the P value is. Smaller trials often lead to lower P values. This is problematic for drugs that exceed the €30 million sales threshold and go through a full benefit assessment. In the United Kingdom, trial size is not a formal criterion, and the respondent noted they can use other data, including natural history, to satisfy budget impact models.
Trial Comparator
In the United States, the comparator is not discussed during the P&T committee reviews, and 21 payers find placebo comparators to be adequate in measuring drug benefit. In France, Germany, and the United Kingdom, the comparator is critical to the review process, and placebo comparators are not always adequate. In France, when there is no comparator, indirect comparisons are made with the input of clinicians. In Germany, placebo comparators are considered appropriate only if no treatment alternative exists or in cases where the treatment is the last line of therapy. In the United Kingdom, indirect comparisons are used in budget-impact models. In the absence of a trial comparator, “do nothing” or “best supportive care” can be considered an appropriate comparator.
Open-Label Extension Data
Open-label extension data are valued by US payers and if they impact the prescribing information or product label, the drug is reevaluated by the P&T committee. In France and Germany, open-label extension data do not affect the reimbursement decision and the German respondent views these data as biased. In the United Kingdom, open-label extension data can improve the reimbursement decision.
Limitations
This research was limited to payers willing and available to participate in market research. In the United States, there are concerns of survey fatigue. In France, Germany, and the United Kingdom, time and budget constraints limited the research to n=1 payer per market. These are representative since these markets have centralized decision making; however, future research should pressure test findings with a broader sample in these markets and in other European markets (eg, Spain and Italy).
Conclusions
To maximize reimbursement of orphan drugs for rare diseases across the United States and Europe, trials should be at least 6 months long, but ideally 12 months to satisfy US payer preferences. Manufacturers can balance this with the fact that payers understand the urgent need to bring treatments to markets and are willing to accept shorter durations than they would for conventional drugs.
Manufacturers should focus on the quality, rather than quantity, of data when it comes to designing trials for orphan drugs for rare diseases. Small sample sizes are common for clinical trials for these drugs; however, payer concerns can be addressed with quality of study design and a demonstration of the large magnitude of patient benefit.
In the absence of a standard of care, placebo trials may be accepted across markets, although in France and the United Kingdom, payers make indirect comparisons. This should not prevent manufacturers from designing placebo-controlled trials, especially in cases where there are no existing treatment alternatives.
Although open-label extension data will not impact the reimbursement decision in France or Germany, they are valued in the United Kingdom and United States and can positively impact market access decisions, even if data become available postlaunch.
Future research should explore how legislative changes in the United States and Europe (eg, the Inflation Reduction Act in the United States and the bill on the Financial Stabilization of the Statutory Health Insurance System in Germany) will impact drug pricing and reimbursement for orphan drugs for rare diseases.
References
1. Nestler-Parr S, Korchagina D, Toumi M, et al. Challenges in research and health technology assessment of rare disease technologies: report of the ISPOR Rare Disease Special Interest Group. Value Health. 2018;21(5):493-500. https://doi.org/10.1016/j.jval.2018.03.004
2. Prescription drug prices in the United States are 2.56 times those in other countries. News release. Rand Corporation; January 28, 2021. https://www.rand.org/news/press/2021/01/28.html
3. Żelewski P, Wojna M, Sygit K, et al. Comparison of US and EU prices for orphan drugs in the perspective of the considered US Orphan Drugs Act modifications and discussed price-regulation mechanisms adjustments in US and European Union. Int J Environ Res Public Health. 2022;19(19):12098. doi:10.3390/ijerph191912098. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9566473/
4. French National Authority for Health. Opinions on medications: ZOKINVY (lonafarnib) 50 mg and 75 mg, capsule; first assessment adopted by the Transparency Committee. French National Authority for Health; 2023. Accessed June 14, 2023. https://www.has-sante.fr/upload/docs/evamed/CT-19943_ZOKINVY_PIC_INS_AvisDef_CT19943_EPI766.pdf
5. Federal Joint Committee (Gemeinsamer Bundesausschuss). Benefit assessment procedure for the active substance lonafarnib (Hutchinson-Gilford progeria syndrome or progeroid laminopathy, from 12 months). Federal Joint Committee; 2023. Accessed June 14, 2023. https://www.g-ba.de/bewertungsverfahren/nutzenbewertung/894/#nutzenbewertung
6. Goldstein S, Runyan A, Merkert J, Schoenwaelder T, Pavic M. HTA184 Investigating how orphan drug manufacturers can optimize clinical trial design so patients across geographies can access life-saving innovations. Value Health. 2022;25(12):S332. doi: https://doi.org/10.1016/j.jval.2022.09.1642
7. Batscheider A, Shlaen E, Rich A, et al. HTA85 possible impact of orphan threshold reduction to 20 million Euro in the framework of early benefit assessment and reimbursement price in Germany. Value Health. 2022;25(12):S312-S313. doi: https://doi.org/10.1016/j.jval.2022.09.1544

