The Importance of Global Collaboration and Knowledge Exchange: A Summary Report of the Global ISPOR HTA Roundtable
Kelly Lenahan, MPH, ISPOR, Lawrenceville, NJ, USA; Jessica Daw, PharmD, MBA, Pharmacy, Sentara Health Plans, Virginia Beach, VA, USA; Mitch Higashi, PhD, ISPOR, Lawrenceville, NJ, USA
Health technology assessment (HTA) bodies and health insurance organizations/payers globally are increasingly being asked to assess innovative health technologies as new drug approvals are on the rise.1,2 The rapid development and introduction of new treatments, particularly advanced therapy medicinal products (ATMPs), gene therapies, precision medicines, and other advanced therapies, present significant challenges for these HTA bodies. These challenges include limited evidence at the time of approval, uncertainty in long-term outcomes, high costs, affordability concerns, equity in access to treatments, and sustainability of healthcare systems. The unique characteristics of advanced therapies, such as their high upfront costs and potential long-term benefits, make assessing their value and affordability particularly difficult, with an additional burden for low- and middle-income countries (LMICs). These countries often face greater difficulty in financing and accessing these high-cost technologies, raising important questions about global equity and healthcare system sustainability.
HTA is a multidisciplinary process that uses systematic and explicit methods to evaluate the properties and effects of a health technology.3 A health technology is an intervention developed to prevent, diagnose, or treat medical conditions; promote health; provide rehabilitation; or organize healthcare delivery. The intervention can be a test, device, medicine, vaccine, procedure, program, or system.4 To improve methodology and information sharing between HTA bodies, ISPOR has been holding HTA Roundtables in 5 major regions of the world annually since 2007 (Asia Pacific, Europe, Latin America and the Caribbean, Middle East and Africa, and North America). These meetings are comprised of HTA bodies, payers and health insurance organizations, other governmental decision makers such as Ministries of Health or the World Health Organization, and academics if no HTA body exists in a country/jurisdiction. However, these meetings have always taken place regionally and not on a global scale. Throughout the years of holding HTA Roundtables, ISPOR continued to hear the need for cross-regional discussion and information sharing. Therefore, for the first time in the 17-year history of the ISPOR HTA Roundtables, ISPOR held the Global HTA Roundtable on October 23, 2024, to bring together the 5 regions to share information and learn from one another.
"To improve methodology and information sharing between HTA bodies, ISPOR has been holding HTA Roundtables in 5 major regions of the world annually since 2007."
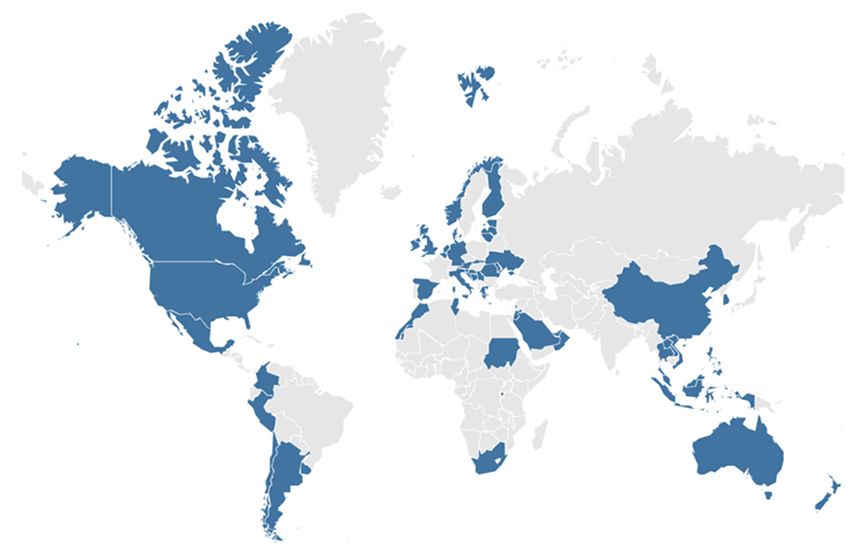
One hundred participants from 53 countries/jurisdictions globally attended the virtual meeting (Figure). A pre-event survey found 3 major aspects of innovative health technologies that participants wanted to discuss: (1) ATMPs/gene therapies/precision medicine, (2) digital health, and (3) high-cost technologies. ATMPs and gene therapies represent a paradigm shift in treatment approaches, often offering potential cures for previously untreatable conditions. These therapies also come with significant challenges in terms of evidence generation, pricing, and healthcare system readiness. High-cost drugs for rare diseases pose unique challenges due to small patient populations and limited evidence, while those for prevalent diseases raise concerns about budget impact and system sustainability. Digital health technologies and artificial intelligence present novel challenges in terms of evidence standards, privacy concerns, and integration into existing healthcare systems. These topics highlight the need for HTA methodologies to evolve to assess these diverse and complex innovations adequately.
Figure. Geographic representation of attendees of ISPOR’s HTA Roundtables.
An overview of the current situation regarding the assessment of these technologies in each region was presented; then participants were divided into 6 breakout groups to discuss the successes and opportunities for each of the 3 major topics listed above. An online survey tool was used to collect responses from attendees for each breakout group. Items identified during the breakouts were then voted on by the entire group. The results of the discussion are below.
ATMPs and Gene Therapies
ATMPs, including gene therapies, represent cutting-edge treatments with the potential to provide curative solutions for previously untreatable conditions. However, these therapies often come with extremely high costs, sometimes reaching millions of US dollars per patient, which places a significant strain on healthcare systems. This cost challenge is exacerbated by the limited available evidence at the time of approval, as well as the small patient populations for many rare diseases. The complexity and high cost of these treatments make it difficult for HTA bodies to assess their value and affordability, particularly in countries with limited resources.
A key concern raised during the roundtable discussions was the significant global disparities in financing and access to ATMPs. For instance, in the United States and Belgium, innovative financing mechanisms, such as value-based contracting and companion diagnostics, have been implemented, but other countries, like Morocco, finance gene therapies on a case-by-case basis. This disparity in access highlights the need for innovative financing models and greater international collaboration to address global inequities in healthcare access.
To reduce costs, European hospitals have expressed interest in developing on-site production for chimeric antigen receptor T-cell therapies (CAR-Ts). On-site production would reduce treatment delays, lower costs, and allow for more personalized development of the technology; however, many regulatory challenges face this manufacturing change due to the complex nature of CAR-Ts. Additionally, joint price negotiations, as seen in the BeNeLuxA initiative, offer a strategy for smaller countries to increase bargaining power and potentially secure more favorable pricing terms. In the United States, value-based contracting for gene therapies is gaining traction, with contracts often extending for several years to ensure the effectiveness of the treatment.
Another challenge facing ATMPs/gene therapies is the limited number of specialized treatment facilities. This is particularly true for newer therapies like those for sickle cell disease. The complex nature of these treatments often requires highly specialized centers with specific expertise and equipment. For example, in the United States, CAR-T cell therapies are typically only available at select cancer centers. This limitation can create significant geographic and logistical barriers for patients who have to travel many hours to receive treatment, leading to potential health disparities. Access issues also impact utilization rates, with some therapies seeing lower uptake than initially anticipated. This situation highlights the need for infrastructure development and healthcare workforce training to expand access to these innovative treatments.
Overall, the Global HTA Roundtable came to consensus that the global HTA community is succeeding by relying on evidence when making reimbursement and coverage decisions and funding through outcomes-based contracts/managed entry agreements. However, there is a strong call for better collaboration between HTA bodies and payers to ensure price transparency and for the development of global frameworks tailored to assessing ATMPs.
"There is a strong call for better collaboration between HTA bodies and payers to ensure price transparency and for the development of global frameworks tailored to assessing ATMPs."
Digital Health Technologies and Artificial Intelligence
Digital health technologies, including artificial intelligence (AI)-enabled systems, present unique challenges for HTA. The rapid pace of technological development and the diverse range of digital interventions make it difficult to create standardized assessment frameworks. Moreover, the dynamic nature of AI, which can learn and evolve over time, adds complexity to its evaluation. Some HTA bodies have developed frameworks to assess these technologies, but they must continually adapt to keep pace with innovation.
AI presents particular challenges related to transparency, the need for specialized expertise, and the integration of AI algorithms into healthcare systems. Many countries are cautiously optimistic about the potential of AI to improve HTA processes, particularly by automating routine tasks and enhancing efficiency. However, there are concerns about the risk of AI exacerbating equity issues between high-income countries (HICs) and low- and middle-income countries (LMICs), as limited data from the latter can lead to biases in AI systems. Furthermore, there is a need to ensure the quality of the data used by AI systems, as inaccurate or incomplete data could undermine the effectiveness of these technologies.
Overall, the Global HTA Roundtable agreed that the HTA community is being successful by being cautious, but open minded, about the use of AI in HTA. The biggest opportunity for improving how the HTA community assesses digital health and AI is the need for standardization of guidelines and methods relating to these types of technologies, including more information sharing of best practices and current guidelines.
High-Cost Technologies
The assessment of high-cost technologies, particularly those used for rare diseases, is another area of significant concern. Innovative payment models, such as pay-for-performance and outcomes-based contracts, have emerged as potential solutions to manage the costs of these treatments. However, these models face significant challenges in terms of implementation, including difficulties in determining when to withdraw treatments that do not meet performance criteria and the need for transparent communication among stakeholders.
In Europe, there has been a shift toward assessing the real clinical benefit of technologies rather than just their cost. This approach aims to prioritize technologies that provide significant patient benefits, particularly in cases where the technology is highly innovative. Canada’s “Drugs for Rare Diseases Strategy” is another example of a proactive approach to managing high-cost treatments, with a financial commitment to subsidizing rare disease drugs and collecting real-world evidence to assess their long-term effectiveness.
Ukraine has successfully implemented Managed Entry Agreements (MEAs) for high-cost drugs. In 2022, 9 medicines went through financial-based agreements, resulting in over $10 million in savings for the healthcare system. These savings have, in turn, enabled access to new therapies that might otherwise have been unaffordable.12 Other countries in central and eastern Europe, such as Moldova, also plan to implement MEAs in the next year.
Argentina’s prioritization system for high-cost drugs, which considers factors such as equity and alignment with public health priorities, is another example of how countries are addressing these challenges. Argentina’s system gives higher priority to technologies that have already been evaluated by agencies in other countries, such as NICE in the United Kingdom or Canadian agencies. This approach allows Argentina to leverage international expertise and streamline its own assessment process, which is particularly valuable given the country’s resource constraints.
Overall, the Global HTA Roundtable came to consensus that the HTA community is succeeding by using price negotiations, managed entry agreements, and using cost-effectiveness thresholds. There is a strong need for information sharing across countries and regions to learn what others are doing and how to share best practices.
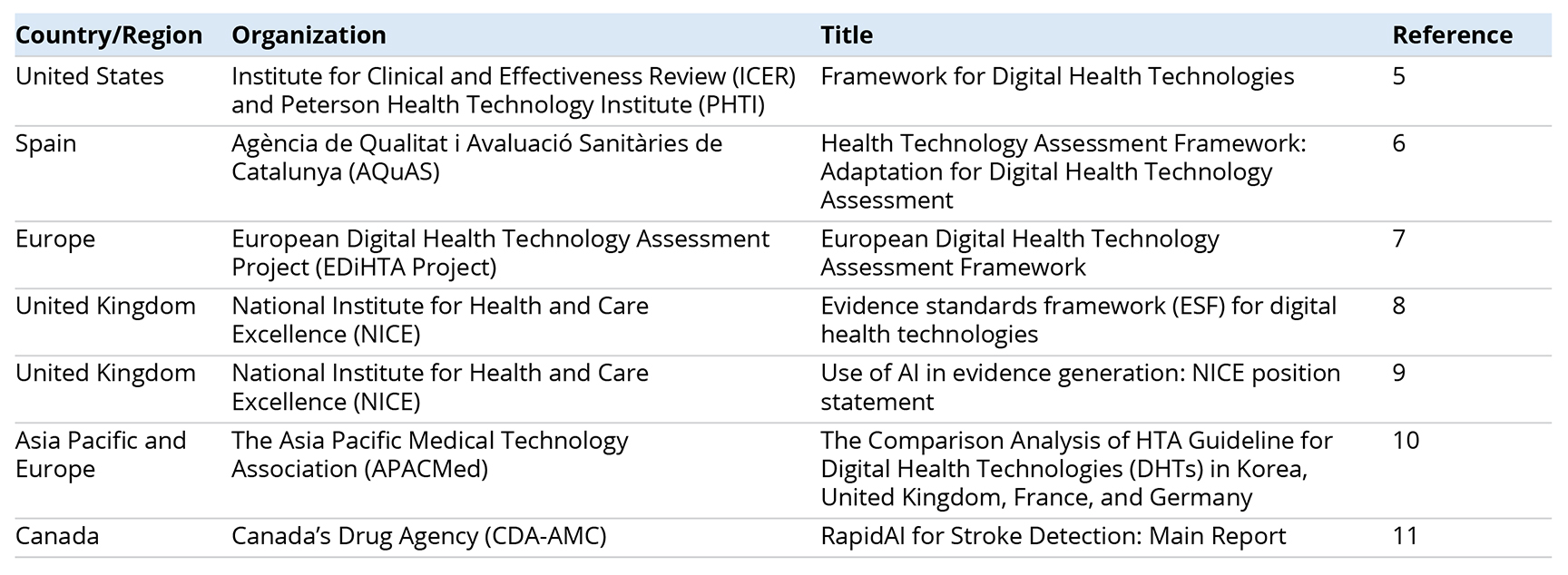
Table. References shared at the Global HTA Roundtable related to the assessment of digital health technologies and artificial intelligence.
Conclusion
The Global HTA Roundtable highlighted the need for greater international collaboration and information sharing to address the challenges posed by innovative health technologies. The discussions underscored the importance of adapting HTA methodologies to assess emerging technologies like ATMPs, digital health, and high-cost treatments. There was a consensus that the HTA community is making progress but must continue to evolve to meet the complexities of these innovations. Key recommendations included (1) improving collaboration between HTA bodies and payers; (2) developing global frameworks for assessing new technologies; and (3) ensuring that the quality of data used in evaluations is robust. Cross-border collaboration and the sharing of best practices were seen as crucial to improving global decision making and ensuring equitable access to life-changing treatments.
References
- de la Torre BG, Albericio F. The pharmaceutical industry in 2021. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2022;27(3):1075.
- Barrie R. FDA beats EMA to most approved new drugs in 2024. Pharmaceutical Technology. https://www.pharmaceutical-technology.com/news/fda-beats-ema-to-most-approved-new-drugs-in-2024/?cf-view&cf-closed. Published January 17, 2025. Accessed January 27, 2025.
- O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. 2020;36(3):187-190.
- HTAGlossary.net. Health Technology. HTA Glossary. https://htaglossary.net/health+technology. Accessed January 27, 2025.
- Institute for Clinical and Effectiveness Review (ICER) and Peterson Health Technology Institute (PHTI). ICER-PHTI Assessment Framework for Digital Health Technologies. https://icer.org/assessment/icer-phti-assessment-framework-fordigital-health-technologies/. Published September 2023. Accessed January 27, 2025.
- Segur-Ferrer J, Moltó-Puigmartí C, Pastells-Peiró R, Vivanco-Hidalgo RM. Health Technology Assessment Framework: Adaptation for Digital Health Technology Assessment. Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQuAS). https://aquas.gencat.cat/web/.content/minisite/aquas/publicacions/2023/framework-adaptation-digital-hta-redets-aquas2023.pdf. Published 2023. Accessed January 27, 2025.
- European Digital Health Technology Assessment Project (EDiHTA Project). European Digital Health Technology Assessment Framework. https://edihta-project.eu/. Accessed on January 27, 2025.
- National Institute for Health and Care Excellence (NICE). Evidence standards framework (ESF) for digital health technologies. https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies. Published December 10, 2019. Updated August 9, 2022. Accessed January 27, 2025.
- National Institute for Health and Care Excellence (NICE). Use of AI in evidence generation: NICE position statement. https://www.nice.org.uk/about/what-we-do/our-research-work/use-of-ai-in-evidence-generation--nice-position-statement. Published August 15, 2024. Accessed January 27, 2025.
- Suh J. The comparison analysis of HTA guideline for digital health technologies (DHTs) in Korea, United Kingdom, France, and Germany. The Asia Pacific Medical Technology Association (APACMed). https://apacmed.org/wp-content/uploads/2020/11/The-Comparision-Analysis-of-HTA-guideline-for-DHTs-in-KOR-UK-FRA-and-GEROct-2020.pdf. Published 2020. Accessed January 27, 2025.
- RapidAI for Stroke Detection: Main Report. Canada’s Drug Agency (CDA-AMC). https://www.cda-amc.ca/sites/default/files/ou-tr/OP0556_RapidAI_for_Stroke_Main_Report_Feedback_Opportunity.pdf. Published August 2024. Accessed January 27, 2025.
- Ministry of Health of Ukraine. For the first time in Ukraine, patients will have free access to medications for spinal muscular atrophy [in Ukrainian]. https://moz.gov.ua/uk/vpershe-v-ukraini-pacientam-budut-bezoplatno-dostupni-liki-proti-spinalnoi-mjazovoi-atrofii. Published December 9, 2022. Accessed January 28, 2025.

