Reimbursement of Digital Therapeutics: Deep Dive on the United Kingdom and Implications for Manufacturers
Victor Moran, MSc, Biotechnology & Business, Precision AQ, London, United Kingdom; David Carr, PhD, Precision AQ, London, United Kingdom; Richard Macaulay, PhD, Precision AQ, London, United Kingdom

Lessons Learned
The United Kingdom’s evolving approach to assess and reimburse digital therapeutics (DTx) offers important opportunities for manufacturers considering launching in Europe. The UK’s Early Value Assessment (EVA) pathway, launched in 2022, demonstrates the government’s intent to promote early adoption of promising DTx, particularly those addressing key national health issues. However, the pathway’s reliance on conditional recommendations, contingent on the collection of real-world evidence (RWE), underscores the need for manufacturers to plan strategically.
One major lesson is the importance of preparing for evidence generation. While EVA facilitates quicker access to the National Health Service (NHS), it requires manufacturers to generate additional data to secure long-term reimbursement. Small- and medium-sized companies must be proactive in securing funding from available UK programs such as the NIHR i4i, NIHR, and OLS RWE Programme to support these efforts.
Another key takeaway is the importance of differentiation to compete in a crowded space. Since the EVA groups DTx in Health Technology Evaluation (HTE) bundles, manufacturers must develop and communicate a compelling value proposition that highlights their product’s unique benefits to stand out in a competitive landscape.
Lastly, the absence of a legal mandate for recommendations to be reimbursed country-wide means that securing NHS reimbursement requires strong relationships with regional NHS entities. Manufacturers should engage these stakeholders early in the process to facilitate adoption and ensure that their DTx achieves the desired access.
In summary, while the United Kingdom offers a promising pathway for DTx reimbursement, manufacturers must be diligent in planning for evidence generation, differentiation, and stakeholder engagement to succeed in this rapidly evolving market.
Digital Therapeutics Offer Huge Clinical Potential but Have Faced Significant Access Barriers to Date
The increasing prevalence of chronic diseases and aging populations are imposing significant challenges on public health systems, with financial pressures expected to intensify in the coming years. Digital therapeutics (DTx) present a promising solution to mitigate these economic challenges to healthcare systems as well as improve health outcomes for patients.
Despite the potential of DTx, many of these technologies are not yet profitable or are only marginally so. In Europe, a major issue is reimbursement by public health systems; demonstration of the clinical value of DTx using traditional health technology assessment (HTA) methods can be very challenging. HTAs rely on randomized controlled trials (RCTs) as the gold standard to demonstrate clinical benefit. However, conducting RCTs for DTx can be impractical due to factors such as difficulty in blinding participants, maintaining patient engagement, and identifying and measuring relevant health outcomes. Further, HTA methods were originally designed for static interventions such as medicine, but digital interventions can rapidly evolve, even more so through artificial intelligence and machine learning, potentially making the intervention more cost-effective over time and necessitating re-evaluations. Accordingly, real-world evidence (RWE) is expected to play an important role in the evaluation of DTx, as it allows continuous assessment of their effectiveness in real-world settings.
There may be, however, notable challenges in the utilization of RWE to support the value of DTx. These include structural barriers that can hinder the collection and utilization of RWE. Many healthcare systems are characterized by fragmented data environments, where information is siloed across different electronic health record systems, registries, and proprietary platforms. These systems often lack interoperability, making it challenging to integrate and harmonize data for analysis. Moreover, variations in data collection practices across regions and institutions further complicate efforts to aggregate comprehensive datasets. Access to data is another obstacle, as policies governing data sharing and privacy may vary widely across jurisdictions. Even when RWE is collected, it often faces inherent limitations when compared to RCTs, such as the introduction of confounding biases due to nonrandomization and concerns about the reliability of data quality (eg, incomplete or omitted registry data). These issues can compromise the generalizability of findings, which may cause payers and HTA bodies to approach RWE with caution.1
"Many healthcare systems are characterized by fragmented data environments, where information is siloed across different electronic health record systems, registries, and proprietary platforms."
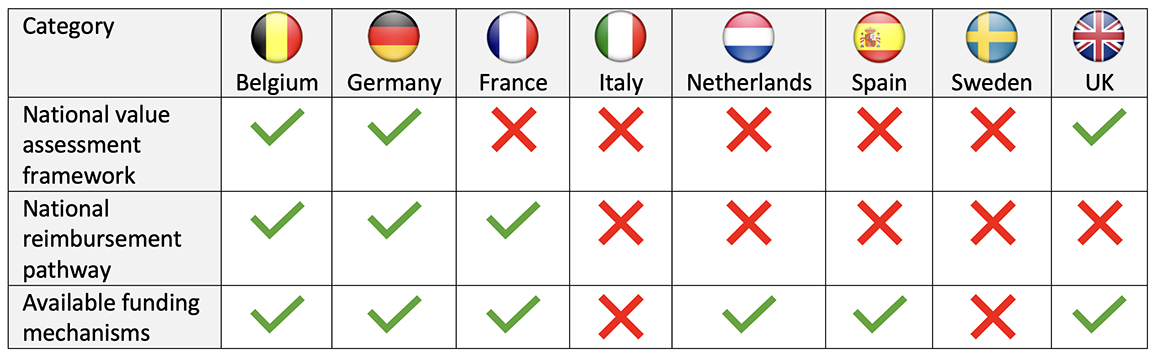
Despite these challenges, the European DTx market size was $1.68 billion in 2023 and is expected to grow to $13.9 billion by 2033.2 The DTx market in Europe is predominantly concentrated in a few nations that have adopted measures to evaluate and/or reimburse DTx (Table). This fragmented and complex market sees Germany as the most influential player, as they were the first in introducing a process for the reimbursement of DTx, the Digital Health Applications (DiGA) in 2020. Under this process, a single RCT is sufficient to obtain reimbursement, decisions are made within 3 months, and the average price for permanently listed DTx is €532.3,4
Table. Overview of the DTx frameworks, pathways, and funding across major European markets
After reimbursement, integrating DTx into the healthcare system may still face challenges, particularly among certain patient demographics. The uptake of DTx may be lower among groups such as those with low digital literacy, older adults, low-income populations, and rural residents. This may be especially concerning as people in these groups typically have greater healthcare needs. These factors influencing DTx uptake may thus hinder equitable adoption and achieving optimal healthcare outcomes.5
The UK Approach: Conditional Reimbursement Pending RWE collection
The UK established an Evidence Review Framework in 2019; however, only one product received a recommendation under this pathway, Sleepio in 2022, at a listed price of £45 per user.3,6 During this period, 29 DTx had been permanently reimbursed in Germany.7
The UK subsequently adopted a distinct approach termed “Early Value Assessment (EVA) for MedTech” in 2022. EVA is designed to facilitate the early adoption of promising technologies addressing national health concerns within the NHS. Through EVA, technologies may receive a conditional recommendation for use, a recommendation for research only, or no recommendation. Those recommended for use will be subject to an evidence generation plan, with a subsequent full NICE assessment based on the new data generated. However, these recommendations have no legal mandate to be followed, although it is expected that they will help support local access.
As of August 7, 2024, 57 DTx had been evaluated by NICE under the EVA pathway in under 2 years. Fifty-two percent of the outcomes were conditional recommendations, 39% for research only, and 9% were not recommended.8
"The European digital therapeutics market size was $1.68 billion in 2023 and is expected to grow to $13.9 billion by 2033."
Considerations for Manufacturers
The EVA pathway has evaluated many DTx in the United Kingdom since its inception in 2022. Most EVA recommendations are conditional on future data collection. However, companies need to find a way to fund these evidence-generation plans. It is critically important, especially for small manufacturers, to leverage various funding programs to support data collection, such as the NIHR i4i, the NIHR, and OLS RWE Programme.
Under EVA, DTx are grouped in Health Technology Evaluation (HTE) bundles comprising multiple technologies with similar characteristics that target the same indication, and often with several DTx securing conditional recommendations. Therefore, competitive differentiation becomes a critical consideration, and development and dissemination of a compelling payer value story centered around the unique product characteristics becomes key.
Finally, the lack of a legal mandate for NICE recommendations under EVA means that manufacturers should establish and leverage strong relationships with local NHS entities early to help secure local reimbursement.
In conclusion, DTx offer the potential for substantial patient and healthcare system benefits, but manufacturers need to plan and prepare appropriately to navigate the rapidly evolving technology appraisal, coverage, and funding dynamics between different markets to ensure these DTx innovations can support healthcare ecosystems in providing timely and appropriate care for patients who so urgently need them.
References:
- Zisis K, Pavi E, Geitona M, Athanasakis K. Real-world data: a comprehensive literature review on the barriers, challenges, and opportunities associated with their inclusion in the health technology assessment process. J Pharm Pharm Sci. 2024;27. 10.3389/jpps.2024.12302
- Europe digital therapeutics market analysis and forecasts, 2023-2033: major players are focusing on innovative product developments and strategic partnerships to strengthen their market position. Yahoo!Finance. https://uk.finance.yahoo.com/news/europe-digital-therapeutics-market-analysis-093200153.html. Published July 4, 2024. Accessed August 7, 2024.
- San Miguel L, Obyn C, Vinck I, de Meester C, Jespers V, Pouppez C. Evaluation of digital medical technologies. Belgian Health Care Knowledge Center. https://kce.fgov.be/sites/default/files/2023-01/KCE_362_Evaluation_Digital_Medical_Technologies_Report.pdf. Published 2023. Accessed August 7, 2024.
- Gensorowsky D, Witte J, Batram M, Greiner W. Market access and value-based pricing of digital health applications in Germany. Cost Eff Resour Alloc. 2022;20(25):1-14.
- van Kessel R, Roman-Urrestarazu A, Anderson M, et al. Mapping factors that affect the uptake of digital therapeutics within health systems: scoping review. J Med Internet Res. 2023;25:e48000.
- Sleepio to treat insomnia and insomnia symptoms. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/mtg70/resources/sleepio-to-treat-insomnia-and-insomnia-symptoms-pdf-64372230458053. Published May 20, 2022. Accessed August 7, 2024.
- Schmidt L, Pawlitzki M, Renard BY, Meuth SG, Masanneck L. The three-year evolution of Germany’s digital therapeutics reimbursement program and its path forward. NPJ Digit Med. 2024;7(139). https://doi.org/10.1038/s41746-024-01137-1
- Carr D, Moran V. NICE Early Value Assessment: a new avenue for evidence generation and early market access in the digital therapeutics space in the United Kingdom. Poster presented at: ISPOR Europe 2024; November 17-20; Barcelona, Spain.

