Individual Reimbursement Approval Under a Managed Access Protocol for Liraglutide (Saxenda®) for Weight Management in Ireland: Design, Implementation, and Outcomes
Claire Gorry, PhD; Stephen Doran, MSc; Rosealeen Barrett, MSc; Michael Barry, MD, Health Service Executive Medicines Management Programme, Dublin, Ireland

Introduction
The authorization of glucagon-like peptide-1 (GLP-1) analogues for weight management has led to a step-change in the treatment of obesity. While these treatments offer promise in terms of improving long-term morbidity and mortality outcomes for patients with obesity, there are significant challenges to their widespread adoption, including uncertainty regarding the optimal duration of treatment, questions around the high cost and affordability of these agents, the ability of manufacturers to provide sufficient product to meet demand, and attitudes regarding obesity as a lifestyle issue rather than a medical condition.1 The high prevalence of obesity and the potential budget impact of widespread adoption of these agents represents a significant financial challenge to publicly funded health systems.2
In Ireland, the Health Service Executive (HSE) has adopted a novel approach utilizing a reimbursement application system (RAS) and a managed access protocol (MAP) to enable access to medicines for weight management in a cost-effective manner. Liraglutide (Saxenda®) is a GLP-1 analogue, and it is licensed as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients, with an initial body mass index (BMI) ≥ 30 kg/m2, or BMI ≥ 27 kg/m2 in the presence of at least one weight-related comorbidity such as dysglycemia, hypertension, dyslipidemia, or obstructive sleep apnea.3 Liraglutide is reimbursed in Ireland by the HSE in a subpopulation of the licensed population, where there is a higher probability of cost-effectiveness, as described below.
An RAS requires the prescriber to submit an individual patient application for reimbursement approval for a medicine. For liraglutide, this is done through an online portal, which is linked to the national pharmacy claims reimbursement system. Applications are reviewed within 3 working days of submission, and for patients who meet the eligibility criteria and are approved, this approval is visible to the prescriber in real-time following a review by a pharmacist member of the HSE-Medicines Management Programme (MMP) team. It is then also immediately visible to the community pharmacist dispensing the medication.
"The high prevalence of obesity and the potential budget impact of widespread adoption of these agents represents a significant financial challenge to publicly funded health systems."
A MAP lists the eligibility criteria attached to reimbursement support for a medicine. In order for an application submitted through the RAS to be approved, the patient must satisfy all of the eligibility criteria as outlined in the MAP. These criteria are developed based on a number of inputs, including the recommendations of the HSE Drugs Group regarding reimbursement conditions, commercial terms as negotiated with the marketing authorization holder (MAH), the product license, pivotal trials, and the health technology assessment (HTA) of the medicine, conducted as part of the pricing and reimbursement process. In this way, a MAP enables evidence-based and cost-effective utilization of medicines. It also provides the decision maker with a greater degree of clinical governance over the use of a medicine, and greater certainty regarding the utilization and expenditure of a medicine post-reimbursement.
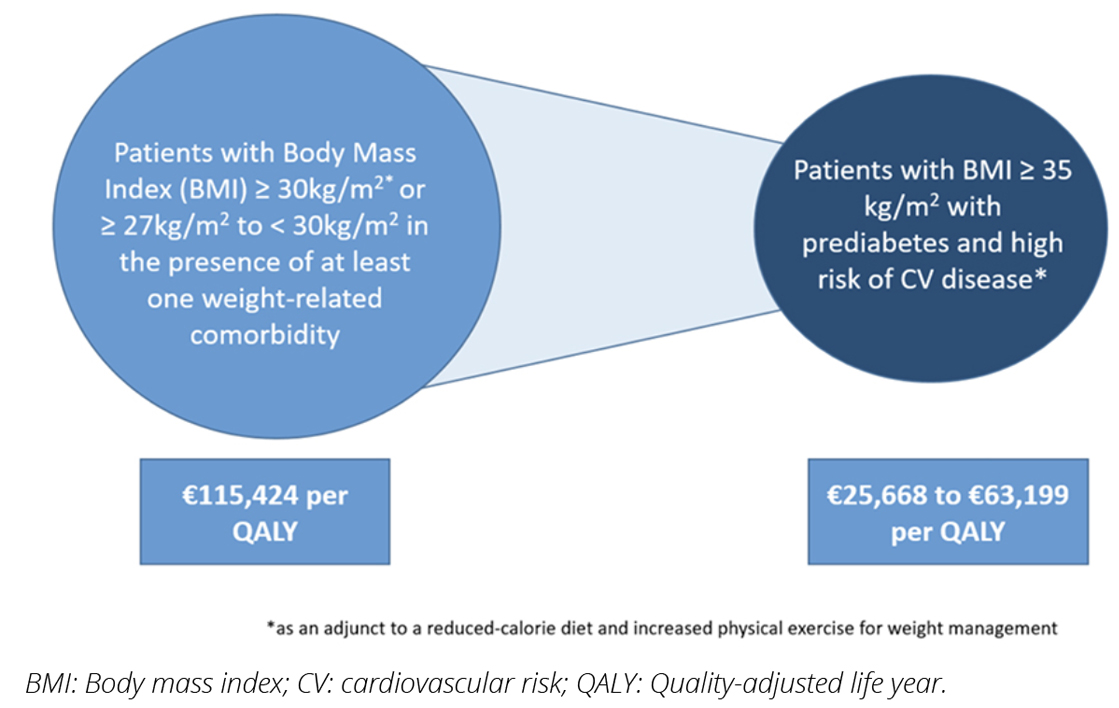
The MAP for liraglutide was developed by the HSE-MMP.4 The HSE Drugs Group recommended reimbursement support for a subpopulation of the licensed population (Figure 1), based on the population assessed in the HTA submission provided by the MAH.5,6 This population consists of patients with a BMI ≥ 35 kg/m2, with prediabetes and high risk of cardiovascular disease, where there is a greater probability of cost-effectiveness (Figure 1). The HSE Drugs Group also recommended that the MAP incorporate guidance on treatment discontinuation for patients who have not lost ≥ 5% of their initial body weight after 12 weeks of treatment with liraglutide 3 mg daily (ie, the maintenance dose), as per the product license.3 This is operationalized via a two-phase approval process, outlined below. Reimbursement is supported for 2 years’ duration, in line with the assumptions in the budget impact model submitted by the MAH.
Figure 1. Outcomes of the cost-effectiveness analysis of liraglutide in Ireland in the full licensed population (left), and the subgroup where reimbursement was recommended by the HSE Drugs Group (right).5,6
To be eligible for phase 1 approval, which provides reimbursement support for 24 weeks (6 months), patients must be aged between 18 and 74 years at time of application. Prescribers must confirm that the patient is actively participating in nonpharmacological interventions for weight management. Patients must have an established diagnosis of nondiabetic hyperglycemia (prediabetes), defined as a fasting plasma glucose of 5.5-6.9 mmol/L and hemoglobin A1c (HbA1c) level of 42-47 mmol/mol, based on readings taken within 30 days of the application. Patients must also be at high risk for cardiovascular disease, defined as fasting total cholesterol > 5 mmol/L or mean systolic blood pressure > 140 mmHg confirmed on a 24-hour blood pressure monitor. For applications where the cardiovascular parameters are outside of those specified, pharmacological management of same is taken into consideration.
There are a number of clinical exclusion criteria for reimbursement support, aligned with the criteria implemented in the SCALE Obesity and Prediabetes trial (NCT01272219) and the Summary of Product Characteristics.3 These include people who are currently pregnant or breastfeeding, have developed obesity secondary to endocrinological or eating disorders, and those with uncontrolled hypothyroidism/hyperthyroidism.
To be eligible for phase 2 approval, the prescriber must submit a phase 2 application providing the patient weight following 12 weeks of treatment with the maintenance dose of liraglutide (3 mg daily). The IT system will automatically determine if this is a weight loss of ≥ 5% compared with the baseline weight and will automatically apply reimbursement approval for responding patients, for up to a maximum of 2 years, treatment duration.
We presented an overview of applications received in the first 4 months of availability of liraglutide in Ireland at ISPOR Europe in November 2023.7 We reviewed the 3081 phase 1 applications for reimbursement support submitted via the online reimbursement application system between January 1 and April 30, 2023. The majority of these applications were for females, with an average age of 50.6 years; 43.5 kg/m2 was the average BMI. High-risk for cardiovascular disease was reported in 83.4% of the applications, and a confirmed diagnosis of prediabetes was reported in 48.2% of the applications. Reimbursement was approved for 1575 (53.4%) applications, as these patients satisfied all the criteria outlined in the MAP.
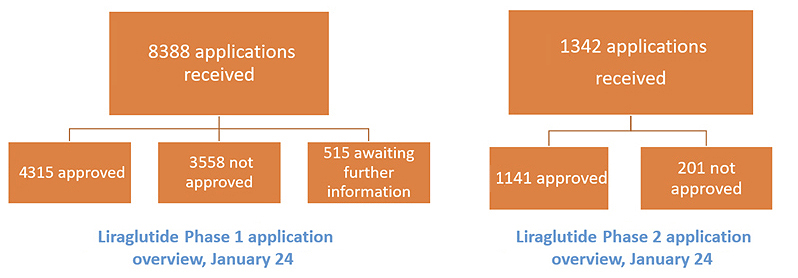
As of January 31, 2024, 8388 phase 1 applications for reimbursement support for liraglutide for weight management had been received, with 4315 patients approved for reimbursement support (Figure 2). Clinicians can submit applications on behalf of any patient; we have not formally analyzed the reasons for the high level of applications submitted on behalf of patients who do not meet the reimbursement criteria. Under phase 2, 1342 applications for reimbursement support had been received, with 1141 patients approved. There is a notable difference in the approval rate between the phase 1 and phase 2 applications; it seems likely that clinicians are mainly only submitting phase 2 applications on behalf of patients who have attained the required weight loss within the time frame.
Figure 2. Overview of applications received via the online reimbursement application system for liraglutide for weight management between January 1, 2023 and January 31, 2024.
In January 2024, 1948 patients were accessing liraglutide under the Community Drug Schemes in Ireland. Cumulative expenditure on liraglutide in this timeframe was almost €3.6 million (including fees and VAT, exclusive of rebates). There have been persistent issues with shortages of liraglutide since the introduction of the MAP, which has undoubtedly had a dampening effect on the cumulative expenditure. The MAP process does provide for an appeals mechanism for phase 1 or phase 2 applications that were not approved; where it can be demonstrated that patients could not access consistent supplies of liraglutide, it has been possible to extend the duration of reimbursement support to allow patients the opportunity to access continuous treatment.
It is possible to consider a scenario where there is no reimbursement application scheme in place, where we assume that all applicants are approved for reimbursement. In this scenario, expenditure over the same period could have been up to €15.4 million. This demonstrates the effectiveness of the MAP in allowing reimbursement support only in the approved cohort where cost-effectiveness has been demonstrated and the power of the MAP to contain expenditure.
Implications and lessons learned
The successful rollout of this health technology management initiative in a high-demand therapeutic area in the primary care setting has allowed eligible patients to access a cost-effective treatment for weight management through their general practitioner. This is in line with the long-term health system reform agenda, Sláintecare, which seeks to deliver care in the primary care and community settings where possible. The implementation of the RAS and MAP for liraglutide will likely have implications for reimbursement of other weight management medicines. The data collected will be available to better inform future reimbursement decisions. This initiative has demonstrated that it is possible to implement outcomes-based conditional reimbursement of a medicine on a national scale through our existing IT infrastructure.
"The successful rollout of this health technology management initiative in a high-demand therapeutic area in the primary care setting has allowed eligible patients to access a cost-effective treatment for weight management through their general practitioner."
The MAP has facilitated patient access while managing the expenditure, as illustrated in our counterfactual expenditure example above. As of December 2023, there were 28 medicines reimbursed subject to a RAS and MAP in Ireland, with more than 9400 applications for individual reimbursement approval reviewed by the HSE MMP in 2023. These medicines cover a broad range of therapeutic areas, are delivered across community and hospital settings, and include medicines like liraglutide with high utilization, but also a number of gene therapy products and other medicines for rare diseases.
Health technology management processes such as RAS and MAPs are now well-established in Ireland.8,9 These processes are enabling patient access to innovative medicines while providing the decision maker with improved oversight and a greater degree of cost certainty, and they are likely to remain part of the reimbursement environment into the future.
Funding Support
No funding support was received for this submission.
Declaration of interest
The authors have no conflicts of interest to declare.
Ethics
The local Research Ethics committee reviewed an ethics application for this study and determined that no ethical consideration/approval was necessary for the analysis of the anonymized data.
References
- Steele M, Finucaine F. Philosophically, is obesity really a disease? Obes Rev. 2023;24(8):e13590.
- Baig K, Dusetzina SB, Kim DD, Leech AA. Medicare Part D coverage of antiobesity medications — challenges and uncertainty ahead. N Engl J Med. 2023;388:961-963.
- Novo Nordisk Limited. Saxenda®
6 mg/ml solution for injection in pre-filled pen. Summary of Product Characteristics. Medicines.ie. https://www.medicines.ie/medicines/saxenda-6-mg-ml-solution-for-injection-in-pre-filled-pen-34785/spc. Updated March 12, 2024. Accessed April 3, 2024. - Health Services Executive Medicines Management Programme. Managed Access Protocol-Liraglutide (Saxenda®) 6 mg/ml solution for injection, as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients, with an initial BMI ≥35 kg/m2 with prediabetes and high-risk for cardiovascular disease. https://www.hse.ie/eng/about/who/cspd/medicines-management/managed-access-protocols/liraglutide-saxenda-/hse-managed-access-protocol-for-liraglutide-saxenda-.pdf. Published May 11, 2023. Accessed April 3, 2024.
- Health Services Executive Corporate Pharmaceutical Unit. HSE Drugs Group September 2021 minutes. https://www.hse.ie/eng/about/who/cpu/drugs-group-minutes/hse-drugs-group-minutes-september-2021.pdf. Published September 2021. Accessed April 5, 2024.
- National Centre for Pharmacoeconomics, Ireland. Cost-effectiveness of liraglutide 3mg (Saxenda®) as an adjunct to a reduced calorie diet and increased physical activity for weight management in adult patients with a body mass index of ≥ 35kg/m2 with pre-diabetes and high risk of cardiovascular disease. https://ncpe.ie/wp-content/uploads/2021/02/Summary-Liraglutide-weight-management-Saxenda-09-02-2021-1.pdf. Published February 2021. Accessed April 3, 2024.
- Gorry C, Daly M, Doran S, Finnigan K, Clarke S, Barry M. Baseline characteristics of patients applying for reimbursement of liraglutide (Saxenda®) for weight management under a managed access protocol in Ireland. Value Health. 2023;26(12):S255.
- Smith A, Barry M. Combining health technology assessment and health technology management to deliver cost-effective prescribing and cost containment-the Irish experience. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):431-436.
- Barrett R, Barry M, McCullagh L. Health technology management: the experience of a managed access approach to the reimbursement of dupilumab in Ireland. Ir J Med Sci. 2023;192(6):2829-2837.

