HEOR Across the Globe

Editor’s Note: Value & Outcomes Spotlight is pleased to introduce “HEOR Across the Globe” as a recurring section in the magazine. The Section Editors work with a small team of Regional Reporters from Asia Pacific, Latin America, and Eastern Europe, Middle East, and Africa to cover developments in health policies, news, and events from these regions. If you have ideas for a story or want to contribute an update, please email voseditor@ispor.org.
ASIA PACIFIC
Section Editor: Paula Lorgelly, PhD, Auckland, New Zealand
Expectations Aplenty for New Zealand’s HTA Agency
Paula Lorgelly, PhD, University of Auckland, Waipapa Taumata Rau, New Zealand
Aotearoa New Zealand’s health technology assessment (HTA) agency, Pharmac, is unique as an HTA agency as it is both the decision maker and the funder of medicines. Their statutory objective is to secure the best health outcomes for eligible people in need of pharmaceuticals within the amount of funding provided. Like many agencies internationally, there has been criticism of the decisions it makes, particularly on how long it takes for medicines to be funded.
In late 2023, Aotearoa New Zealand held a general election in which Pharmac’s functions and processes were featured in every main political party’s manifesto. The proposals ranged from promises of ring-fenced funding for cancer drugs to replacing Pharmac with a new patient-focused medicines buying agency. Pharmac featured in both coalition agreements and the National-led coalition government created a new ministerial portfolio specifically for Pharmac. Most recently Pharmac received its instructions (or expectations) from the Minister regarding how it is expected to perform (indeed reform).
The Letter of Expectations lists 21 specific requests for Pharmac to consult on and ultimately take action. These include reviewing the dual role of value assessment and procedure, updating methods to include the wider fiscal impacts of funding or not funding medicines and the availability of tools to consider the wider societal impact, and considering how those with lived experience can be part of the decision-making process.
The Chair of Pharmac has accepted these expectations, and work is ongoing with both the board and the agency to understand the impact of these expectations. At the October 2024 Board meeting, Pharmac’s dual role as decision maker and funder was discussed and the advice back to the Minister will be for these roles to remain colocated.
There is much interest in how Pharmac will address the expectation to consider the wider fiscal/societal impact. Currently, the perspective employed in economic evaluation evidence is that of the health system, the costs and cost savings for Pharmac, and the impact on primary and secondary care. There are several different ways to expand the evaluation space, and HTA agencies around the world differ in their scope. Previous National governments have promoted a social investment approach to understand the value and impact of interventions in the social services space. How this approach could be aligned with an economic evaluation using cost per quality-adjusted life year is yet unknown.
Watch this space! Will Aotearoa New Zealand offer up another unique HTA perspective? And will it address the long-held criticisms of the agency?
Health Insurance Reform in South Korea
Sang-Soo Lee, PhD, MBA, Medtronic North Asia (Korea and Japan), Seoul, South Korea
A forum was held on 9 January 2025 to discuss elements of South Korea’s public and private health insurance reform. Reform is needed to address systemic inefficiencies, reduce financial burdens on citizens, and ensure sustainable healthcare delivery
for all.
The National Health Insurance Service (NHIS), South Korea’s single national payer, presented its proposals to improve the management of noncovered services. The primary goals are to ensure that essential treatments currently excluded are covered, while strengthening oversight of excessive or overused noncovered services.
Key measures include expanding coverage by way of transitioning essential treatments—such as surgical materials—into the scope of insurance coverage based on cost-effectiveness. NHIS is also looking to introduce managed coverage, a new category for noncovered services prone to overuse. This managed coverage will include new treatment guidelines and pricing criteria. Finally, NHIS is considering limiting coverage for nonmedically necessary services (eg, cosmetic surgeries) when performed alongside insured treatments. A final proposal for the national health insurance is to regularly reassess noncovered services to help eliminate treatments with questionable efficacy or safety, ensuring better resource allocation and patient care.
At the same forum, the Financial Services Commission (FSC) shared plans to reform private insurance with the aim of addressing medical overuse, correcting market imbalances, and reducing unnecessary utilization of minor noncovered services by a small percentage of users. The FSC is seeking to align copayment rates, focusing on severe illnesses (eg, cancer and cardiovascular diseases), while limiting coverage for overused noncovered services and improving private insurance disclosures to build trust and ensure fair practices.
The wider discussions at the forum emphasized the need to reallocate savings to protect essential medical services and restoring balance within the healthcare system. The next steps involve refining the proposals given the stakeholders’ input from the forum, with detailed plans to be announced in the near future.
Eastern Europe, Middle East, and Africa
Section Editor: Bertalan Németh, PhD, Budapest, Hungary
Data Classification in Egypt
Gihan Hamdy Elsisi, MSc, PhD, The American University in Cairo, Egypt
Egypt’s healthcare system faces several current challenges regarding data classification. One major challenge is the lack of standardized data classification frameworks, which hinders the effective management and sharing of healthcare data across various parties.
This figure shows the different public data types, uses, and its multiple sources (Egyptian Authority for Unified Procurement, Medical Supply, and Technology Management; Universal Health Insurance Authority; Egypt Healthcare Authority; Ministry of Health; Health Insurance Organization; Egyptian Drug Authority; Program of Treatment at the Expense of State; Non-Governmental Organizations; Contract Research Organizations).
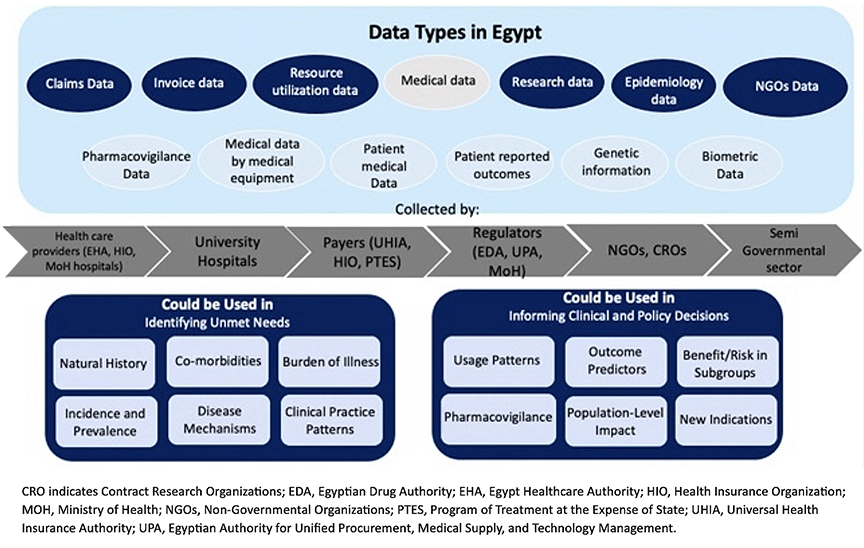
Claims data, medical data, epidemiology data, and nongovernmental data could be used in identifying the unmet needs of disease natural history and comorbidities, incidence and prevalence of different diseases, clinical practice patterns, and disease mechanisms. These data could also be used in informing clinical and policy decisions in usage patterns, outcome prediction, benefits/risks in different subgroups, pharmacovigilance, and new indications.
Invoice data, research data, and resource utilization data could be used to identify the unmet needs of burden of diseases and also could be used in informing policy decisions in population-level impact and identifying the benefits/risks in different subgroups. It is important to classify data to be able to share it among different stakeholders and make informed decisions.
What Is the Cost of Vision Impairment and How Can We Reduce It? A Call to Action for Bulgaria
Maria Dimitrova, PhD, Alexander Oscar, PhD, Zdravko Kamenov, PhD, Maria Kamusheva, PhD, Konstantin Tachkov, PhD, Radka Goranova, PhD, Kostadin Angelov, PhD, Alexander Simidchiev, MD, Zornitsa Mitkova, PhD, Guenka Petrova, DSc, Medical University of Sofia, Bulgaria; Petia Stratieva, MD, PhD, Retina Bulgaria, Sofia, Bulgaria; Rosen Dimitrov, MSc, NRetia Health, Sofia, Bulgaria
Recent studies have shown that in Bulgaria, age-related macular degeneration constitutes a huge economic burden. Key factors contributing to the total economic burden are direct medical costs and productivity losses due to impaired or lost vision.
In 2024, an expert meeting organized by the Bulgarian Association of Pharmacoeconomics and the ISPOR Bulgarian Chapter discussed a strategic direction with a proposal for specific activities aimed at creating a National Eye Care Program and a National Prevention Center that would focus on the early detection of vision problems in both children and adults. The experts called for a comprehensive approach to eye health and making it a priority area. They agreed that implementing current recommendations for prevention (ie, screening and early diagnosis), as well as timely treatment of eye diseases according to modern standards, should start from early childhood.
This issue will be further discussed between the ISPOR Bulgaria, responsible institutions and key opinion leaders. Patient awareness programs will be organized to strengthen prophylactics.
First International Conference on Drug Pricing Policy in Serbia
Guenka Petrova, DSc, Medical University of Sofia, Bulgaria
A new international conference on drug pricing, The Influence of Pricing Policy on Drug Availability, will be held on 28 March 2025 in Belgrade, Serbia.
The conference gathered together representatives of various stakeholders from the European Union and Serbia, as well as from neighboring Adriatic countries. The main aim of the conference was to create a platform for discussion on the new regulations, share experience gathered in different countries, and to propose measures to improve patients’ access to and affordability of medicines.
The topics of the conference covered hot issues related to medicine availability and affordability in Central and Eastern European Countries. The drug pricing policies in Montenegro, North Macedonia, Bosnia and Hercegovina, and Serbia were discussed in the first plenary session. The second plenary session was dedicated to economic factors influencing drug availability. The last session was devoted to cooperation between the countries in the region.
Latin America
Section Editor: Diego Rosselli, MD, Bogotá, Colombia
Colombian Healthcare System Faces Challenges Amid Reforms
Diego Rosselli, MD, EdM, MSc, Pontificia Universidad Javeriana, Bogotá, Colombia
Colombia’s healthcare system, long considered a success story in Latin America, is experiencing significant changes that have raised concerns among medical professionals and patients alike.
The system, established in 1993, achieved universal coverage for the country’s 50 million inhabitants and maintained low out-of-pocket expenses. It provided access to treatments for rare diseases, chronic conditions, and high-cost cancer drugs, often surpassing the capabilities of neighboring countries.
During the COVID-19 pandemic, the system demonstrated its strength by covering expensive treatments and achieving high vaccination rates. However, recent reforms have begun to alter the landscape of healthcare delivery in Colombia.
The core of the health system’s financing, previously a combination of public and private insurers reimbursed through a capitation-based scheme, is undergoing modifications. The new approach aims to have health providers receive direct payments from the government with streamlined audit processes. These changes have led to uncertainty in the healthcare sector.
The immediate effects of these reforms are now becoming apparent. Several small- and medium-sized clinics have closed their doors, particularly impacting obstetrical and pediatric services. Patients are experiencing longer wait times for specialist appointments and surgical procedures. Some individuals requiring orphan drugs or chemotherapy have reported difficulties in accessing these treatments. Healthcare professionals are also feeling the impact of these changes, with many expressing worry about the system’s ability to maintain the quality of care previously provided.
As Colombia navigates this period of transition in its healthcare system, the medical community emphasizes the importance of ensuring continued access to quality care for all citizens. The coming months will be crucial in determining the long-term effects of these reforms on the nation’s healthcare landscape.

