Training Patients to Be Effective Stakeholders in the EU HTA Process
Finn McCartney MA, Aikaterini Charapa MA, Maria Dutarte MA, Sarah Bernier MA and HTA4Patients Project Consortium, EUPATI, Utrecht, The Netherlands
What is the EU HTA regulation?
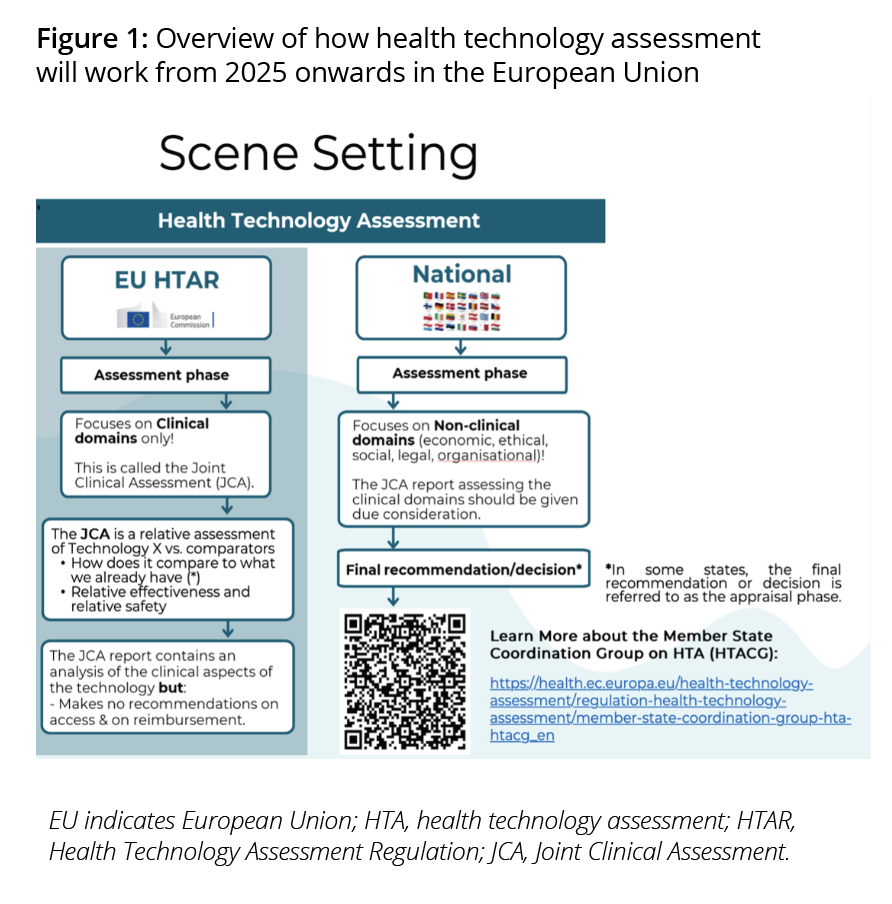
The process of how health technologies are assessed in the European Union (EU) is changing; the EU Health Technology Assessment Regulation (HTAR) will come into effect in 2025 with the aim of harmonizing and simplifying the clinical assessment of a given health technology via Joint Scientific Consultations (JSC) and Joint Clinical Assessments (JCA).
These joint assessments will be performed by an appointed subgroup of members from the Health Technology Assessment Coordination Group (HTACG), which includes a group of representatives from the Member States. The regulation means that cooperation across the European Union is mandated and that one centralized system will be in place for health technologies to be assessed. This has the additional benefit of eliminating the need for duplicating work for multiple national HTA processes (normally a health technology developer would submit the same information to several HTA bodies separately). Also, the produced JCA reports must be given due consideration by a given member state in their respective national HTA processes for a given health technology (Figure 1).
What does this mean for patients in the EU?
The EU HTAR has made patients and patient organizations key stakeholders that will need to be consulted throughout an EU HTA. There are several mechanisms and opportunities for these stakeholders to provide input in this process at the national and European levels.
"The regulation means that cooperation across the European Union is mandated and that one centralized system will be in place for health technologies to be assessed."
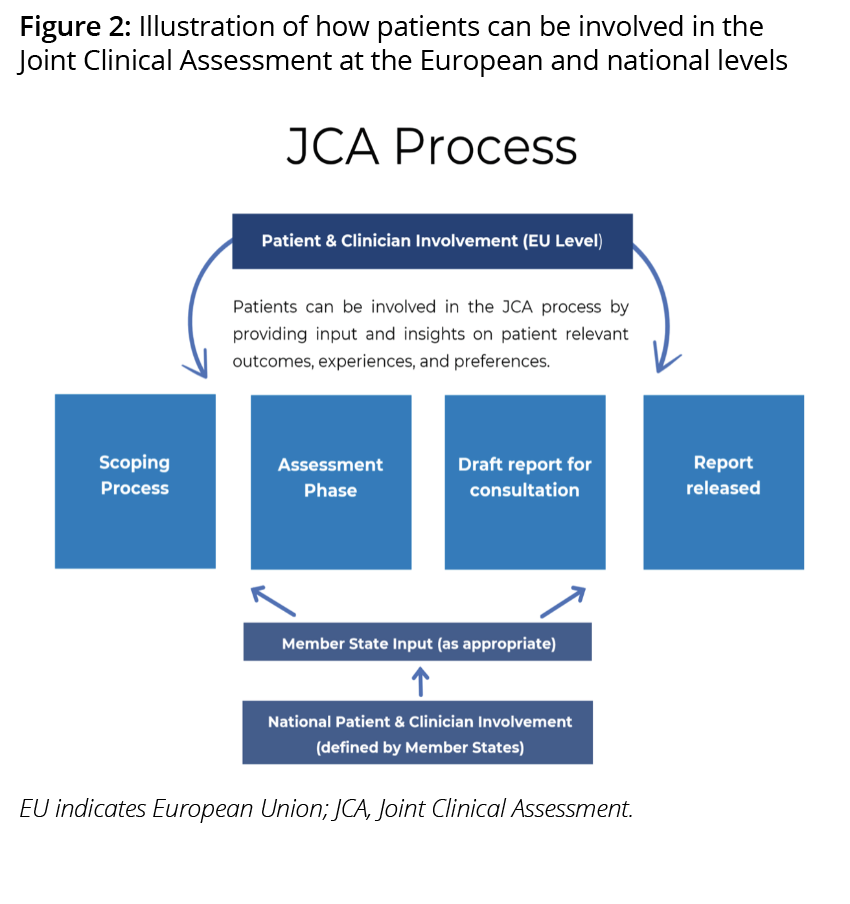
In the JCA, illustrated in Figure 2, patients will be involved in the scoping process (PICO questions) and they will be able to provide input in the draft (JCA) report. These steps will be crucial for patient involvement since patients can provide insights of their own experiences, needs, and perspectives or the multiple experiences, needs, and perspectives of the patient group(s) they represent.
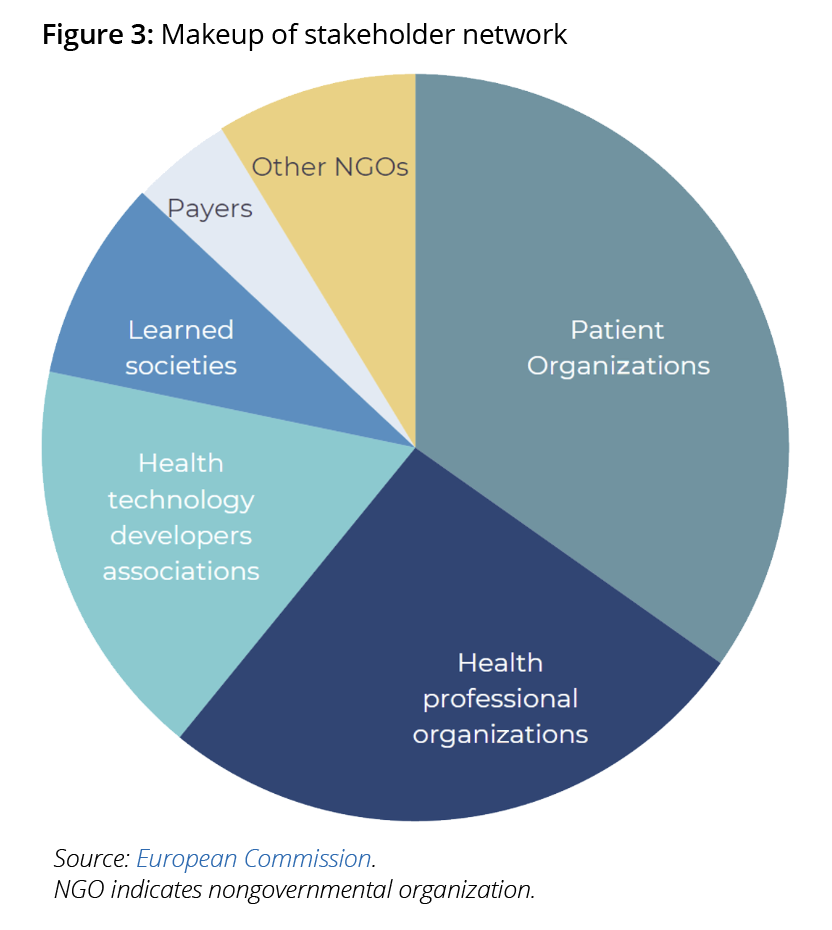
As of 2023, patient organizations are also the largest group represented in the Stakeholder Network—a formal part of the regulation that facilitates dialogue between European umbrella stakeholder organizations and the HTACG.
Patient organizations in this network (Figure 3) will play a crucial role in providing input to the HTACG’s annual work program, annual report, and identifying patient experts who will be relied upon during the joint work (JSC & JCA). This is one of several mechanisms and opportunities for these stakeholders to provide input in this process at the national and European levels.
What are the remaining challenges that patients’ have identified?
The European Patients’ Academy on Therapeutic Innovation (EUPATI) is committed to providing information and training on health innovation to patients and patient representatives, focusing on their involvement in these processes, including HTA. In 2023, EUPATI launched an EU-funded project, HTA4Patients, focusing on building knowledge among patient communities around the HTAR.
Within the framework of this EUPATI project, when interacting with these groups, patients have expressed their difficulty in accessing such a complex topic like HTA: how it works and relates to their personal treatments and patient journey, why does it matter, and the technical jargon associated with this topic.
EUPATI has observed a clear gap in the knowledge and capacity about the EU HTA process and the subject of HTA more generally. For patients to be fully informed, they need training on what is changing in the EU HTA landscape and how they can prepare for this new reality.
What resources can patients access to learn more about the EU HTAR?
To build capacity within the patient community, the European Commission (EC) (HADEA/EU4Health) is funding the above-mentioned EUPATI HTA4Patients project as well as EUCAPA (coordinated by EURORDIS) with the goal of producing educational resources and training on the new regulation.
Through the HTA4Patients Project, EUPATI and its partners have cocreated a free e-learning course, EU Health Technology Assessment Regulation (HTAR), and will be organizing online training sessions on the HTAR for patient groups. Both these materials were created by authoring groups comprising representatives of EUPATI Fellows (patient experts), partners (including ISPOR), EUPATI National Platforms, and other members of its wider network. Since its inception, EUPATI has used this multistakeholder approach to put patients at the center of the discussions and guarantees that our resources are both scientifically reliable and in patient-friendly language.
"To build capacity within the patient community, the European Commission is funding EUPATI’s HTA4Patients with the goal of producing educational resources and training on the new regulation."
In each lesson of the online course, the learner is taken through each element of the process and the information has been presented in a variety of forms to engage the reader. At the end of the course, the learner can take an assessment to obtain a certificate that they have completed the course. Like all EUPATI’s courses, they are freely accessible and can be accessed anywhere in the world. This is one of many ways patients can prepare themselves for the EU HTA process.
EUPATI is now in the process of planning for the online training sessions that will run from October 2024 until September 2025. Patient organizations interested in having EUPATI run a tailored training session for them can express their interest here.
Also, the online course and training materials will be translated into Czech, French, Greek, German, and Spanish by summer 2025. These translations will maximize the reach of these training materials in the patient community.
As of August 2024, the English version of the course has already been accessed by over 2500 unique learners. This shows that the patient community is already preparing themselves for 2025 and it is critical that they are made aware and receive training on the EU HTA process now and as it evolves.
How can patients prepare for 2025?
In addition to training, there are several ways patient stakeholders can prepare themselves for the EU HTAR. The following is a checklist for patients, patient representatives, and patient organizations prepared by EUPATI as part of the HTA4Patients project:
(1) Strengthen your network:
- Check the EMA’s list of eligible patient organizations
- Connect with relevant patient organizations
- Check who is in the stakeholder network
- Connect with EUPATI national platform
(2) Build your capabilities:
- Familiarize yourself with HTA fundamentals
- Familiarize yourself with EUCAPA and HTA4Patients training
- Learn more about PICOs
- Get familiar with conflict of interest examples
- Sign up for relevant newsletters
- Visit the EC’s latest updates
(3) For patient organizations:
- Get to know the HTAR and legislative framework
- Familiarize yourself with the regulatory frameworks for marketing authorizations (EMA)
- Become a member of the stakeholder network, if eligible
- Participate in public consultation on the Implementing Acts
- Organize training for your members
Continued commitment to patient involvement
HTAR presents a challenge but also an opportunity for patient involvement in HTA. In partnership with ISPOR and other members of its multistakeholder network, EUPATI is committed to enhancing efforts within health literacy and patient education in the area of HTA. Through an effective, innovative, and collaborative model of cocreation and codelivery of training, long-standing impact can be created in this space.
Acknowledgments:
We would like to acknowledge that the HTA4Patients materials for the online course and training sessions were made possible due to the work carried out by members of the Authoring Group, Training Group, Editorial Board, Project Management Group & Project Expert Panel.

