Impact of Nirsevimab for All Infants in Preventing Respiratory Syncytial Virus-Related Hospitalizations and Costs in the Brazilian Private Healthcare System
Maicon Falavigna, HTA unit, Inova Medical, Porto Alegre, Brazil; Sarah F Watanabe, Sanofi, São Paulo, Brazil; Juliana Santoro, Sanofi, São Paulo, Brazil; Karina Ribeiro, Sanofi, São Paulo, Brazil; Aline Tolardo, Sanofi, São Paulo, Brazil; Nayê Balzan Schneider, HTA unit, Inova Medical, Porto Alegre, Brazil; Marco Aurélio Palazzi Safadi, Department of Pediatrics, Santa Casa de São Paulo School of Medical Sciences, São Paulo, Brazil; Renato T. Stein, School of Medicine, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil; Alexia Kieffer, Sanofi, Lyon, France

Implications
Respiratory syncytial virus (RSV) remains a significant cause of morbidity and healthcare burden, particularly in infants. While preterm infants and those with comorbidities face higher individual risks of RSV-related hospitalizations, >80% of these hospitalizations occur in healthy full-term infants. This underscores the importance of strategies to prevent RSV in the broader population. This study showed that nirsevimab can substantially reduce both the burden of RSV-associated hospitalizations and healthcare costs in the Brazilian private healthcare system, preventing 5647 hospitalizations, including 1699 pediatric intensive care unit (PICU) admissions, resulting in savings of BRL 61.5 million with inpatient care.
"There is still an evident lack of nationwide hospitalization and mortality data related to RSV-associated LTRD, highlighting difficulties in defining the priorities and investments needed for prevention and treatment."
Introduction
RSV is the most common cause of LRTD in infants and younger children and contributes to substantial morbidity and mortality worldwide. The virus mainly infects children in the first few years of life, with around 50% of children infected during the first year of life and almost all by the age of 2 years.1 Among children infected during the first year of life, it is estimated that 30% to 70% develop LRTD.1 In 2019 exclusively, RSV was responsible for an estimated 33 million acute LRTD globally, resulting in 3.6 million hospital admissions and 26,300 in-hospital deaths in children aged under 5 years.2 Although premature infants and those with underlying comorbidities are among those at highest risk for severe illness, most hospitalizations due to RSV occur in healthy infants born at term.3
In Brazil, RSV was detected in 23% to 61% of infants hospitalized for LRTD.4 However, there is still an evident lack of nationwide hospitalization and mortality data related to RSV-associated LTRD, highlighting difficulties in defining the priorities and investments needed for prevention and treatment.
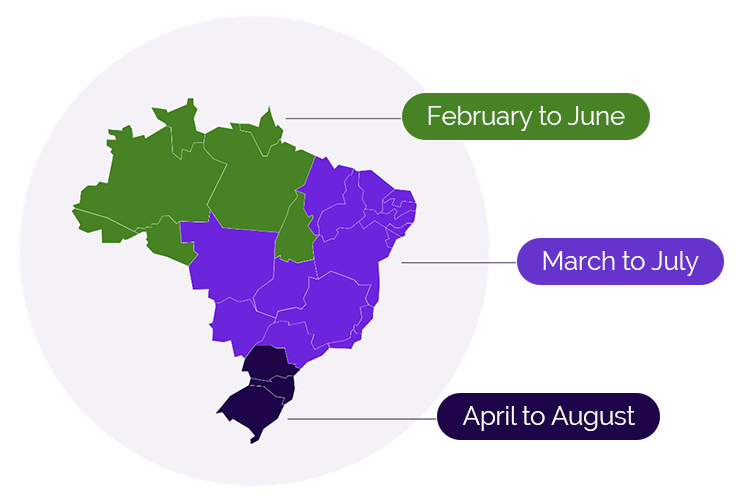
The seasonality of RSV varies from temperate to sub-tropical countries and from the Northern Hemisphere to the Southern Hemisphere. Brazil, a continental nation, is the world’s fifth-largest country by area and has 3 distinct RSV seasons (Figure 1).5 The season typically begins early in the country’s North region in February, followed by the Midwest, Southeast, and Northeast regions starting in March, and finally, the South region beginning in April. On average, each season lasts about 5 months.
Figure 1. RSV seasonality in Brazil
Currently, prophylaxis with the monoclonal antibody palivizumab is the only option available in the private Brazilian healthcare system for preventing RSV infection. However, this option is limited to a restricted group: children under 1 year old born prematurely (with a gestational age of up to 28 weeks), children up to 2 years old with lung disease of prematurity or pulmonary dysplasia, and children up to 2 years old with significant congenital heart disease.6 This leaves a large number of children, especially those up to 1 year of age born at more than 28 weeks gestation, without prophylactic coverage, relying only on infection control measures and supportive therapy. Subsequently, a significant portion of the population remains unprotected against the virus. This includes children with greater vulnerability such as immunocompromised children, children with cystic fibrosis, Down’s syndrome, and other conditions not currently covered.
Nirsevimab is a single-dose monoclonal antibody for passive immunization with an extended half-life, which has 70-80% efficacy for the prevention of RSV-associated LRTD in broad infant populations, regardless of gestational age. Nirsevimab has emerged as a safe and effective alternative for preventing lower respiratory tract infections associated with RSV, meeting the need for protection for a wider range of children.7
"The present study aimed to evaluate modeled RSV-related hospitalizations and costs of the impact of nirsevimab on the private healthcare system compared to standard of practice."
Private healthcare in Brazil consists of private out-of-pocket services and a large private health insurance market. With over 48 million users, representing 24.9% of the Brazilian population, private healthcare plays a significant role in the country’s total health expenditure.8 The present study aimed to evaluate modeled RSV-related hospitalizations and costs of the impact of nirsevimab on the private healthcare system compared to standard of practice (SoP).
Methods
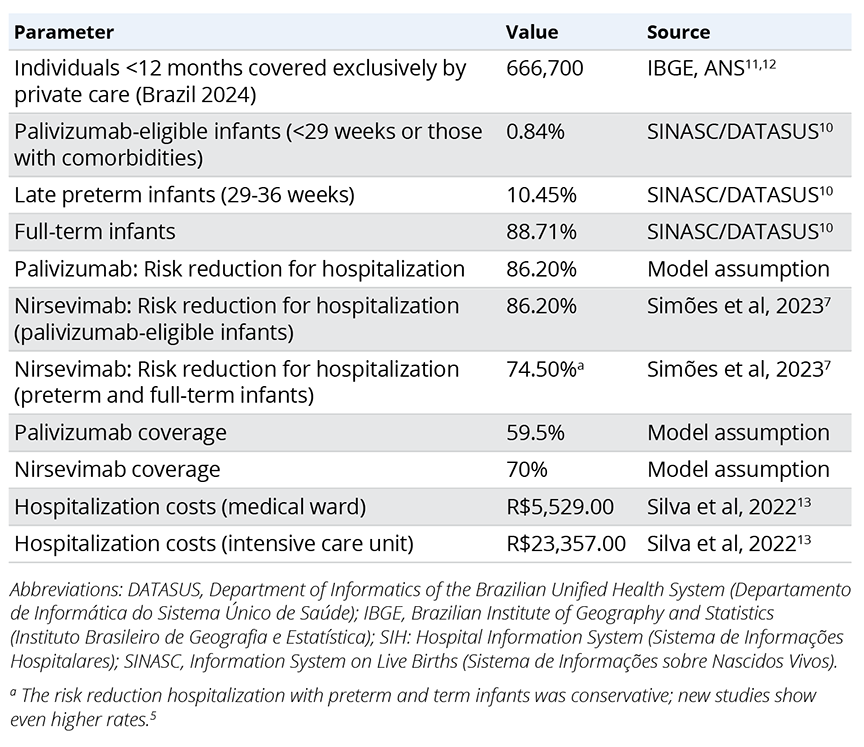
A decision analytic model was used to estimate RSV-associated LRTD events in a Brazilian birth cohort during their first year of life, considering the private payer’s perspective (Figure 2).9 Model parameters were derived from published literature and national databases (Table 1).7,10-13 RSV hospitalization rates were based on Hospital Information System from the Department of Informatics of the Brazilian Unified Health System (SIH/DATASUS),10 adjusted for gestational week at birth according to published literature (Figure 3).14 Intensive care unit (ICU) admission risk was based on published literature. Based on Brazilian market data, we assumed a prophylaxis coverage rate of 70% with nirsevimab and 59.5% with palivizumab (corresponding to a mean of 4.25 doses received by 70% of infants). To be conservative, the risk reduction for hospitalization for palivizumab was assumed to be the same as nirsevimab achieved in published studies.
Figure 2. Decision analytic model structure
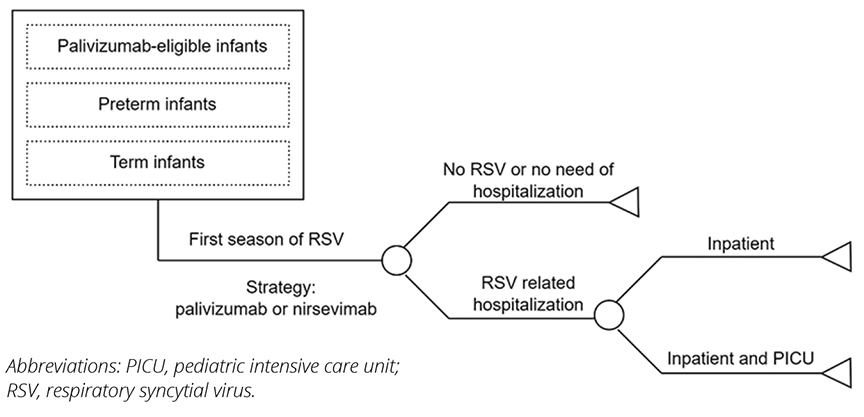
Figure 3. Annualized respiratory syncytial virus-related hospitalization rate by gestational age (A) and risk of intensive care unit admission among hospitalized patients (B)
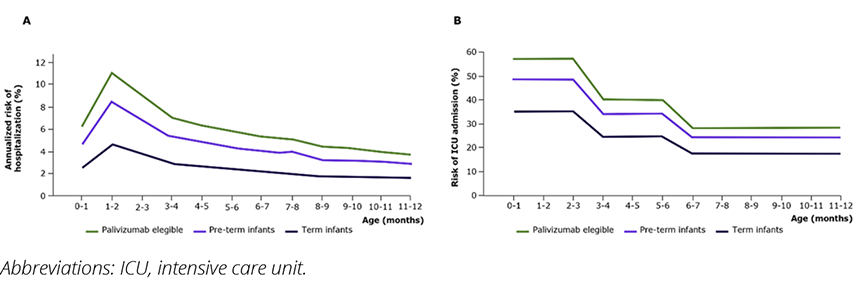
Table 1. Model parameters
Results
This model predicts that 58.1% of RSV-related hospitalizations occur during the RSV season, while the remaining 41.9% occur outside the season (Figure 4). Based on the SoP, it is estimated that there will be 201 RSV-related hospitalizations for palivizumab-eligible infants, 2779 for late preterm, and 13,086 hospitalizations for full-term infants. Additionally, the model predicts 4712 admissions to the PICU due to RSV.
Figure 4. Number of hospitalized patients by gestational age (A) and number of intensive care unit–hospitalized patients by gestational age (B), and hospitalizations costs (in BRLa) by gestational age
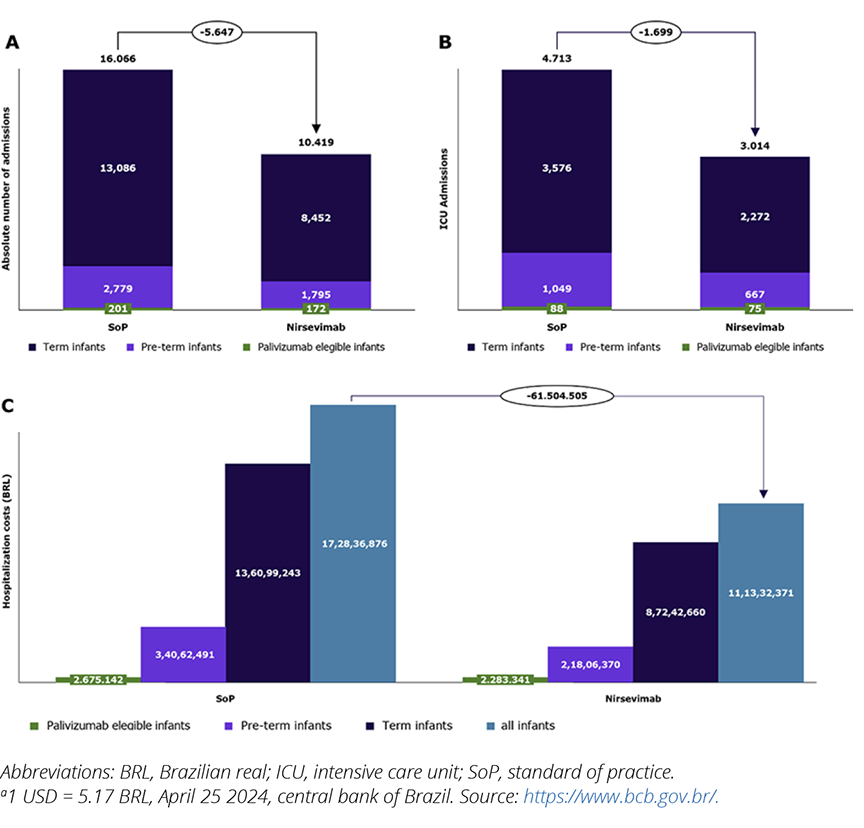
In the modeled scenario, nirsevimab prevented 5647 hospitalizations, with 29 in palivizumab-eligible infants, 984 in late preterm infants, and 4634 in full-term infants. These prevented hospitalizations also included 1699 PICU admissions.
The model suggests that by preventing RSV-related hospitalizations, nirsevimab had the potential to save nearly Brazilian real (BRL) 61.5 million. This includes BRL 0.4 million for palivizumab-eligible infants, BRL 12.3 million for late preterm infants, and BRL 48.9 million for full-term infants (Figure 5). These savings do not account for outpatient care and social costs.
Figure 5. Univariate sensitivity analysis. (A) variation in savings; (B) variation in the number of hospitalizations prevented
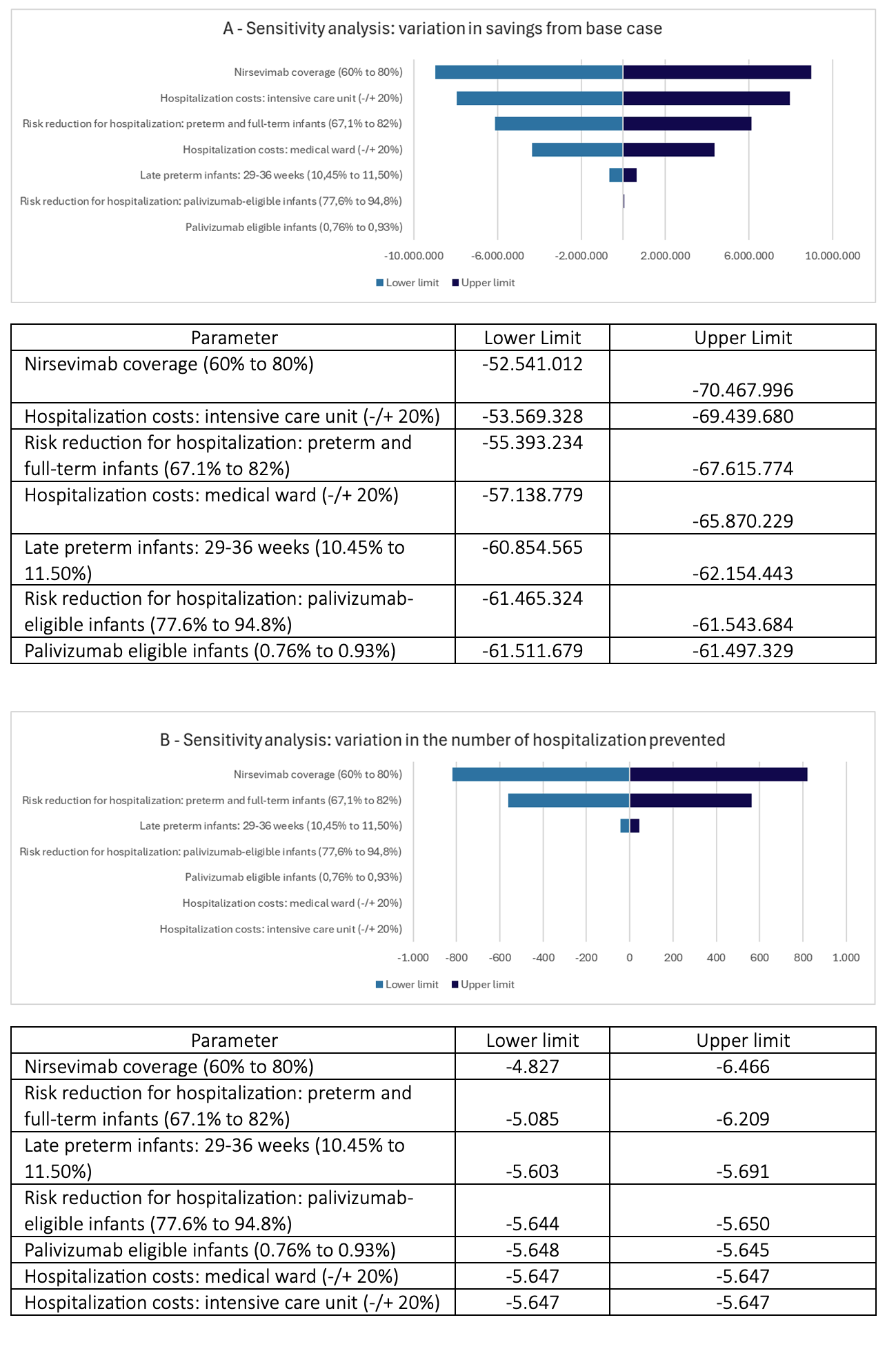
Sensitivity analysis showed consistent results when different coverage rates were applied. An absolute reduction of 10% in the estimated coverage of nirsevimab (from 70% to 60%) would result in savings of BRL 52.5 million and prevent 4827 hospitalizations (including 1450 in the ICU); an absolute increase of 10% (from 70% to 80%) would result in savings of BRL 70.5 million and prevent 6466 hospitalizations (including 1947 in the ICU).
Conclusions
While preterm infants have a higher individual risk of hospitalization, term infants account for nearly 80% of the total number of RSV-related hospitalizations. This underscores the importance of strategies to prevent RSV in the broader population.
Nirsevimab is designed to provide long-lasting protection to all infants, regardless of gestational age. After developing this analysis, other studies were published confirming that nirsevimab has superior efficacy in reducing the risk of hospitalization.5,15-17
"Nirsevimab is designed to provide long-lasting protection to all infants, regardless of gestational age."
Nirsevimab could substantially reduce RSV-related hospitalization burden and costs. This study offers information for policymakers and healthcare managers to evaluate the potential benefits of reimbursing nirsevimab into RSV prophylaxis strategies. Further research is necessary to assess the impact of nirsevimab in the Brazilian private setting.
Funding
This study was sponsored by Sanofi and AstraZeneca. Nirsevimab is being developed and commercialized in partnership between Sanofi and AstraZeneca.
Conflicts of Interest
SW, JS, KR, AT, AK are Sanofi employees, and may hold stock and/or stock options in the company. MF and NBS received professional service fees from Sanofi for conducting this research. MAPS and RTS participated in advisory board for Sanofi.
Acknowledgement
The authors thank Manojkumar Patel, Sanofi, for the medical writing assistance. The authors were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
References
- Binns E, Tuckerman J, Licciardi PV, Wurzel D. Respiratory syncytial virus, recurrent wheeze and asthma: a narrative review of pathophysiology, prevention and future directions. J Paediatr Child Health. 2022;58(10):1741-1746.
- Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047-2064.
- Munro APS, Martinón-Torres F, Drysdale SB, Faust SN. The disease burden of respiratory syncytial virus in Infants. Curr Opin Infect Dis. 2023;36(5):379-384.
- Lamarão LM, Ramos FL, Mello WA, et al. Prevalence and clinical features of respiratory syncytial virus in children hospitalized for community-acquired pneumonia in northern Brazil. BMC Infect Dis. 2012;12:119.
- Staadegaard L, Caini S, Wangchuk S, et al. Defining the seasonality of respiratory syncytial virus around the world: National and subnational surveillance data from 12 countries. Influenza Other Respir Viruses. 2021;15(6):732-741.
- Aprova o protocolo de uso do palivizumabe. Ministério da Saúde. Gabinete do Ministro. http://bvsms.saude.gov.br/bvs/saudelegis/sas/2013/prt0522_13_05_2013.html. Published May 13, 2013. Accessed May 18, 2023.
- Simões EAF, Madhi SA, Muller WJ, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc Health. 2023;7(3):180-189.
- Silva B, Hens N, Gusso G, Lagaert S, Macinko J, Willems S. Dual use of public and private health care services in Brazil. Int J Environ Res Public Health. 2022;19(3):1829.
- Kieffer A, Beuvelet M, Sardesai A, et al. Expected impact of universal immunization with nirsevimab against RSV-related outcomes and costs among all US infants in their first RSV season: a static model. J Infect Dis. 2022;226(s5uppl 2):S282-S292.
- BRASIL. Ministério da Saúde. DATASUS. Sistema de Informação sobre Nascidos Vivos. 2023. https://datasus.saude.gov.br/informacoes-de-saude-tabnet/.
- Agência Nacional de Saúde Suplementar. TabNet Linux 2.6a: beneficiários por operadora [Internet]. ANS Tabnet. Brasília; 2023. http://www.ans.gov.br/anstabnet/cgi-bin/dh?dados/tabnet_cc.def
- Projeções da População. Instituto Brasileiro de Geografia e Estatística. https://www.ibge.gov.br/estatisticas/sociais/populacao/9109-projecao-da-populacao.html. Accessed May 18, 2023.
- Silva GT MR, Regis MR, Paiva APL. Use of health resources and micro-costing analysis of hospitalization for bronchiolitis and pneumonia in pediatric patients: a retrospective study from Brazilian perspective [abstract]. Value Health. 2022;25(12):S148.
- McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36(11):990-996.
- López-Lacort M, Muñoz-Quiles C, Mira-Iglesias A, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Eurosurveillance. 2024;29(6):2400046.
- Ernst C, Bejko D, Gaasch L, et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Eurosurveillance. 2024;29(4):2400033.
- Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73:209-214.

