A Review and Comparative Case Study Analysis of Real-World Evidence in European Regulatory and Health Technology Decision Making for Oncology Medicines
Zong J, Rojubally A, Pan X, et al. Value Health. 2025:28(1):31-41
Section Editor: Agnes Benedict, Executive Director, Evidera Health Economics & Market Access
Contributor: Kerry Winter, Research Associate, Evidera Health Economics & Market Access
Several European bodies have issued guidance regarding the use of real-world evidence (RWE) in regulatory and health technology assessment (HTA) decision-making, reflecting its growing importance in healthcare.1-5 Zong et al set out to review the use of RWE by the European Medicines Agency (EMA) and major HTA bodies, including the National Institute for Health and Care Excellence (NICE), Gemeinsamer Bundesausschuss (G-BA), and Haute Autorité de Santé (HAS), in their evaluations of oncology medicines to describe trends and compare usage across these institutions.
Zong et al manually retrieved oncology European public assessment reports (EPARs) from the Committee for Medicinal Products for Human Use reviews for oncology medicines with marketing authorizations between January 2020 and December 2022, then conducted single-timepoint searches for NICE, HAS, and G-BA. Noncancer, biosimilar, and terminated assessments were excluded. The authors analyzed trends in RWE usage, types of RWE considered, and acceptance levels across these agencies. They also included case studies on how RWE for specific medicines was evaluated by different HTA bodies.
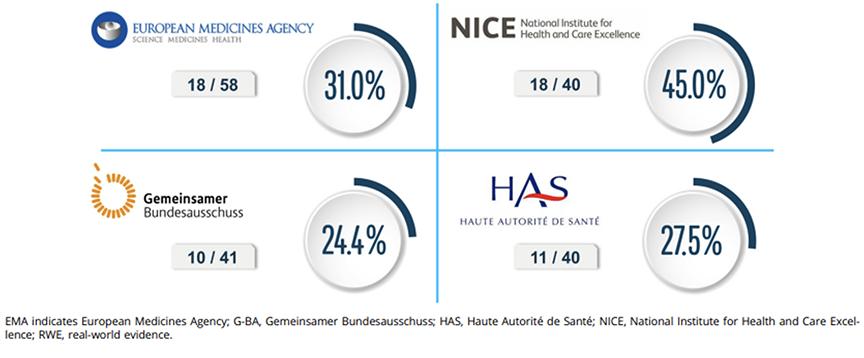
The findings indicate that RWE is becoming significant in oncology decision making. EMA included RWE in 31% of its oncology assessments, with usage increasing over 3 years. Among HTA bodies, NICE had the highest uptake, referencing RWE in 45% of appraisals, compared to 27.5% for HAS and 24.4% for G-BA. The Figure illustrates these differences, with NICE leading in RWE incorporation.
Figure. Extent of RWE included in final approvals and appraisals. Extent of RWE was reported as the percentage of appraisals/approvals including RWE over the total number of appraisals/approvals identified for oncology medicines during the study time periods.
Significant differences were found in how agencies consider and apply RWE. NICE and HAS were more likely to accept RWE as supporting evidence, especially for contextualizing clinical trial results, while G-BA often rejected RWE due to perceived flaws in study design or relevance to the German healthcare context. EMA took a balanced approach, recognizing RWE’s value but calling for follow-up studies to address limitations.
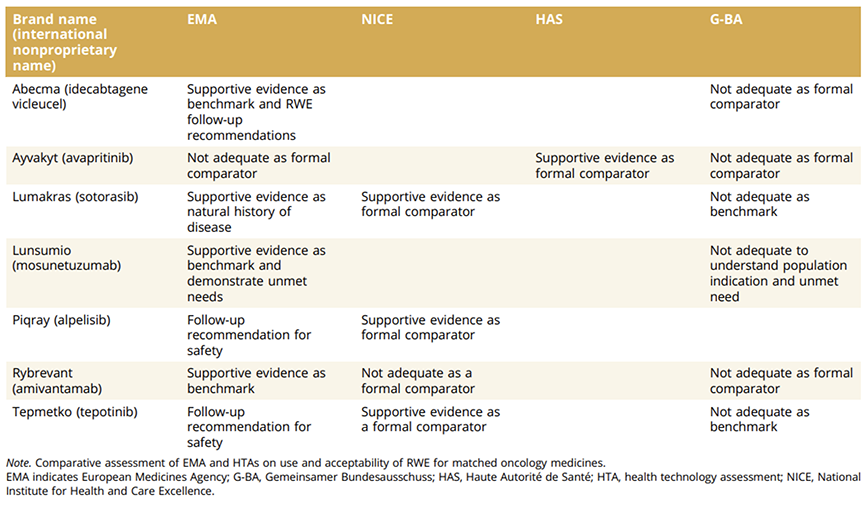
The results highlight the potential and challenges of integrating RWE in oncology decision making. The included Table, comparing RWE acceptability for specific medicines across EMA and HTA bodies, underscores the variability in assessments. For example, NICE accepted RWE as supportive evidence for Lumykras (sotorasib), while G-BA found it insufficient. These differences reveal diverse priorities and criteria, emphasizing the need for greater compatibility.
Table. Case studies of RWE acceptability across EMA and HTA bodies.
Such inconsistencies may hinder the wider adoption of RWE, undermining its potential to make regulatory and HTA processes more efficient. The study suggests driving consistency in standards to enhance RWE’s value for accelerating access to innovative therapies. The EU Joint Clinical Assessment in 2025 presents an opportunity for progress.
A key feature of the study is its structured comparative approach, offering readers a comprehensive overview of RWE usage among major health decision makers in Europe. Comparative case studies covering various cancer areas illustrate how differing agency priorities and methodologies impact RWE acceptability. Limitations include language barriers and potential differences in interpreting agency comments. Future research could explore RWE usage over a longer timeframe or examine how specific methodologies affect decision-making outcomes.
This review and analysis by Zong et al can inform ongoing discussions about RWE’s role in regulatory and HTA decision making, highlight progress and areas for improvement, and set goals for advancing RWE use in oncology and beyond.
References
- A vision for use of real-world evidence in EU medicines regulation. European Medicines Agency. https://www.ema.europa.eu/en/news/vision-use-real-world-evidence-eu-medicines-regulation. Published November 24, 2021. Accessed January 18, 2025.
- Guideline on registry-based studies. European Medicines Agency. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-registry-based-studies_en.pdf. Published October 22, 2021. Accessed January 18, 2025.
- NICE real-world evidence framework. National Institute for Health and Care Excellence. https://www.nice.org.uk/corporate/ecd9/chapter/overview. Published June 23, 2022. Accessed January 18, 2025.
- Real-world studies for the assessment of medicinal products and medical devices. Haute Autorité de Santé. https://www.has-sante.fr/upload/docs/application/pdf/2021-10/real-world_studies_for_the_assessment_of_medicinal_products_and_medical_devices.pdf. Published June 10, 2021. Accessed January 18, 2025.
- Concepts for the generation of routine practice data and their analysis for the benefit assessment of drugs according to §35a social code book V (SGB V). Institute for Quality and Efficiency in Healthcare (IQWiG). https://www.iqwig.de/download/a19-43_routine-practice-data-for-the-benefit-assessment-of-drugs_rapid-report_v1-0.pdf. Published January 10, 2020. Accessed January 18, 2025.

