What Will the Legislative Proposal for Joint Work on European Health Technology Assessment Approach Mean for Access in Europe?
Sabina Heinz, BSc (Hons), MSc, DPhil, Ipsos GmbH, Munich, Germany; Ekaterina Bondal, BSc (Hons), MPhil, Ipsos, London, England, United Kingdom; Samantha Morrison, BSc (Hons), Ipsos, London, England, United Kingdom
Introduction
After considerable discussions, the European Commission voted in March 2021 to adopt the regulation that would see joint clinical assessments on health technologies across Europe.1 This will provide valuable scientific information to national health authorities, harmonize the approach to assessment across Europe, remove redundancies in the system, and provide support to countries that may lack the resources or infrastructure to undertake such assessments on their own. Joint clinical assessments (JCAs) are at the core of the health technology assessment (HTA) regulation. JCAs will be limited to the clinical comparative review of the technology, while member states remain responsible for economic aspects of HTA, drawing conclusions on added value for their health system, and making decisions on pricing and reimbursement.2
"Overall, the survey results indicate that the HTA regulation may potentially improve alignment between countries and increase equality in access to therapies across Europe."
The transition period was set for 3 years to ensure preparedness from all stakeholders. Therefore, JCAs and joint scientific consultations will begin assessments, initially for oncology products and advanced therapy medicinal products, in January 2025. The next stage will include orphan drugs in 2028, with medicinal products from other therapeutic areas following 2 years later.2
In contrast to medical products, the joint work for medical devices will not follow the progressive implementation approach. Instead, the focus will be on high-risk medical devices (eg, class III implantable devices). A coordination group formed of expert panels will decide which medical devices to start with (the evaluation of low-risk medical devices will remain at a national level). The JCAs for the medical devices in scope will not start before 2030.3
After the initial agreement in March, Ipsos fielded an online survey in June 2021, with 35 payers from the Ipsos payer panel (eg, France, Germany, Italy, and Spain) and 13 respondents with global/European remit for market access at multinational pharmaceutical companies. The goal was to gather stakeholder views on the proposal.
Discussion
While the member states will be obliged to use JCAs for qualifying medical products and devices, additional clinical and nonclinical assessments will be permitted, while national HTA assessments for drugs not in scope will be required to run concurrently.2 This brings into question whether the regulation would actually improve the availability of innovative technologies and the equality of access for patients across Europe. As certain therapies will continue to be assessed via national process until 2030, making comparisons across therapy areas potentially challenging given the differences in process and focus between countries and their JCA approach. As agreed in the development of JCA, the decision whether to fund and the acceptable price level to enable funding will remain a matter for the countries to decide.2 Hence, the impact of JCAs—while standardizing the clinical assessment process—may not result in the ultimate goal of equality of access in Europe. This was highlighted in our survey as a key driver for stakeholders, as access will be discussed and agreed on a country level depending on the value to the member state’s healthcare system.
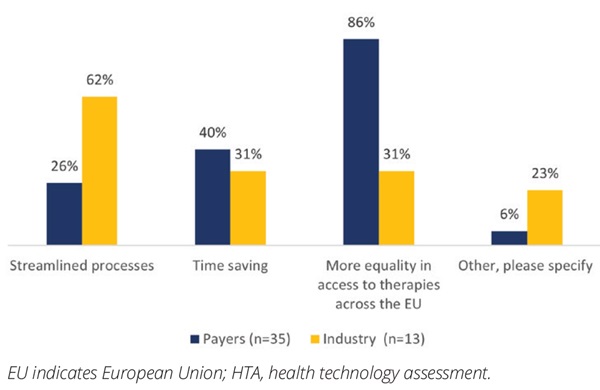
In line with the goals of JCAs, our payers saw greater equality to access across Europe as a key advantage of the process (Figure 1). Industry respondents also expected streamlined processes; however, it should be noted that they appeared much less informed about the changes. Equally, the proposals resulted in some concerns from both the payer and industry perspective, with the areas of disadvantage noted being lack of country control/perspective from the payers (Figure 2). Industry respondents were most concerned about the duplication of HTA processes and the additional administrative burden, in addition to the lack of country control. Despite concerns, the majority of payers (86%) felt this provided an opportunity to ensure more equality of access across Europe.
Figure 1: Perceived benefits of a joint clinical HTA process
As oncology and advanced therapy medicinal products will be the initial focus of the program, there are immediate areas that will require alignment including, but not limited, to:
• Differing standard of care
• Acceptance of endpoints
• Performing indirect comparison
• Assessing the added benefit with no direct comparator
• Dealing with single-arm studies
Figure 2: Perceived disadvantages of a joint clinical HTA process

Although EUNetHTA has previously done work developing methods for the joint assessments, those were not meant to replace national HTA assessments. Since member states require different information for decision making, the disparity in requirements among member states is an impediment to the development of a joint submission dossier template. Other organizational challenges include developing a template for JCAs that is tailored to the needs of each member country, as well as defining the scope of the JCA.
What EUnetHTA 21 & Heads of Agencies Group are doing to address these challenges
EUnetHTA 21 joint consortium will provide support to the future European HTA system to be established according to the upcoming regulation. The consortium is led by ZIN (The Netherlands) and includes 12 EU HTA agencies.4
Member states already carried out some joint assessments in the past, which were performed alongside the usual national HTA assessments, and previous analysis indicated these had little impact on the national processes. This was demonstrated by the time it took to obtain reimbursement following an EUnetHTA assessment, which varied by member state, implying that member states primarily focus on meeting national requirements when evaluating new medicines.5 Given the progressive implementation of the regulation, the national HTA system will have to keep ensuring the adequate assessment of other drugs. Both industry respondents and payers noted additional administrative burden and expanded time to approval as disadvantages of the joint clinical HTA process (Figure 2). The need to continue running standard national-level assessments of drugs outside the initial scope of the JCAs—while also designating members for a Coordination Group that would carry out JCAs and joint scientific consultations—is likely to become a resourcing challenge, potentially delaying patient access to new medicinal products.
A recently formed initiative, the Heads of Agencies Group,6 will work during the next 3 years alongside the EUnetHTA 21 joint consortium to support the implementation of the HTA regulation. Current members of the group include 19 national authorities involved in HTA activities. The group will focus on supporting the preparation of national systems and capacities, as well as championing the work performed by the technical and scientific collaborations of HTA bodies across Europe. In addition, it will advise policy makers and national organizations on matters related to HTA.5 It remains to be seen whether such collaborative efforts will be sufficient to tackle prospective resourcing issues of member states and thus ensure timely HTA assessments.
JCA dossier submission timeline
JCAs are subject to strict timelines to ensure that access to medical products is not hampered in member states. To meet the demanding timelines, the regulation requires health technology manufacturers to submit JCA dossiers at the latest 45 days prior to the envisaged date of the opinion of the Committee for Medicinal Products for Human Use (CHMP), while the Coordination Group is to endorse draft reports no later than 30 days following the marketing authorization.2 However, starting HTA work before a medical product receives market authorization is quite controversial because the product may not receive a positive opinion from CHMP, meaning all work done by the Coordination Group up to this point has been a misallocation of precious joint resources. In addition, the recommendations for the final treatment label and specific conditions for use are granted by CHMP, which means that some critical aspects for assessment are not affirmed at the time of JCA dossier submission.
Will smaller countries be willing to accept the compromises required more readily than those with well-established HTA systems?
Member states that did not have an established HTA system or resources in place might be more willing to accept the format, guidelines, and methodologies of the regulation to improve the assessment of medicinal products brought to their markets. Many have already shown the willingness to look outward.
“The adoption of this law is another demonstration of how EU countries, when acting together, can achieve very practical results for their citizens. This new law will benefit patients, producers of health technologies, and our health systems,” said Janez Poklukar, the Slovenian minister for health.
In contrast, countries with more established HTA systems might be less willing to compromise on country-specific regulations and principles in favor of JCAs. This means that, following the JCA process, member states may impose complementary clinical analysis for national HTA processes—potentially delaying patient access to new therapies, a concern highlighted in the survey results (Figure 2).
Given that the regulation’s focus is clinical assessment, the nonclinical (economic) assessment will be left to the national HTA process. This leads to the question of how, if at all, JCAs would affect a country’s ability or willingness to reimburse and thus influence equality of access.
Conclusion
Overall, the survey results (as well as positive views from member states) indicate that the HTA regulation may potentially improve alignment between countries and increase equality in access to therapies across Europe. The collaborative efforts are already taking place to address methodological and organizational hurdles. For instance, members of EUnetHTA 21 are working on development of draft methodological guidelines to be adopted by the Coordination group, while members of the Heads of Agencies Group are focusing on supporting the preparation of national systems. Consequently, despite the existing challenges, the collaborative work and engagement of all member states provide JCAs with the potential to improve equality of access for patients across European markets (which is in the interests of all stakeholders) and streamline processes for the industry and member states.
References
1. European Commission. Health Technology Assessment: Commission Welcomes The Adoption Of New Rules To Improve Access To Innovative Technologies.; 2021. Accessed April 26, 2022. https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771.
2. European Parliament and the Council of the European Union. Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU; 2021. Accessed January 13, 2022. https://eur-lex.europa.eu/eli/reg/2021/2282/oj.
3. Giorgio, F. Together for HTA In Europe [Conference presentation]. HAS-PFUE Symposium, France. March 7, 2022.
4. EUnetHTA. EUnetHTA 21. 2021. Accessed April 26, 2022. https://www.eunethta.eu/eunethta-21/.
5. Chew C, Sogokon P, McNamara L, et al. The Effect of EUnetHTA Assessments on National HTA Decision Making. Presented at: ISPOR Europe 2018; 10-14 November, 2018; Barcelona, Spain.
6. EUnetHTA. European HTA Agencies Launch the Heads of Agencies Group (HAG).; 2021. Accessed April 26, 2022. https://www.eunethta.eu/european-hta-agencies-launch-the-heads-of-agencies-group-hag.

