Health Equity within Health Technology Assessments: What Progress Has Been Made?
Lydia Yejin Lee, PharmD, MS, Rutgers Ernest Mario School of Pharmacy and Rutgers School of Public Health, Piscataway, NJ, USA and GSK, Collegeville, PA, USA; Sunny Sheth, PharmD, MBA, RPh, Rutgers Ernest Mario School of Pharmacy and Rutgers School of Public Health, Piscataway, NJ, USA and Novo Nordisk, Plainsboro, NJ, USA; Samantha N. Valliant, PharmD, Rutgers Ernest Mario School of Pharmacy and Rutgers School of Public Health, Piscataway, NJ, USA and GSK, Collegeville, PA, USA; Zeba M. Khan, PhD, RPh, Rutgers Ernest Mario School of Pharmacy and Rutgers School of Public Health, Piscataway, NJ, USA
Introduction
Health technology assessment (HTA) bodies have the unique and influential role of evaluating health technologies to create policies that affect healthcare resource access and allocation.1 HTA allows evidence-based decision making that aims to support equitable and effective healthcare systems.2 Although various health equity-related frameworks have been developed to support the fair and systematic incorporation of health equity into HTA decision making, there is a lack of consensus on how to incorporate health equity measures.3-5 This article delves into a review presented at ISPOR Europe 20236 aimed at understanding the extent of health equity incorporation in global HTA report of cystic fibrosis medicines shedding light on critical gaps and potential recommendations.
The review included HTAs conducted by HTA bodies and independent organizations conducting these assessments (eg, Institute for Clinical and Economic Review [ICER]). National HTA bodies were identified using the International Network of Agencies for Health Technology Assessment. Identification of the HTA reports was conducted searching the following terms using the Boolean operator “OR:” cystic fibrosis, lumacaftor, elexacaftor, tezacaftor, ivacaftor. A targeted literature review was conducted to determine an appropriate, relevant framework to assess the HTA reports. The Framework for Equity by Benkhalti et al5 was used to review included HTA submissions to evaluate the extent of health equity incorporation within these HTAs. Some considerations included stakeholders’ involvement, outcome measures, and methodological approach.
"Although various health equity-related frameworks have been developed to support the fair and systematic incorporation of health equity into HTA decision making, there is a lack of consensus on how to incorporate health equity measures."
Key Findings
Among the 33 HTA bodies identified, only 12 had reports on cystic fibrosis medicines, with 3 meeting the inclusion criteria. The review revealed varying degrees of health equity consideration among the included reports. While the Canadian Agency for Drugs and Technologies in Health6 and the Institute for Clinical and Economic Review7 demonstrated patient and advocacy group engagements, there was limited consideration of medication access and its impact on patient outcomes. For instance, CADTH allowed information to be submitted directly from patient groups on outcomes and issues important to patients and caregivers and directly engaged advocacy groups. ICER utilized formal questionnaires and reports from the US Food and Drug Administration in addition to direct patient and advocacy group engagement.8 European HTA had patient and organization inclusion frameworks, but the extent of incorporation was not clear in the submission analyzed.
Recommendations for Improvement
Several recommendations were identified to enhance the integration of health equity in HTA assessments (Figure). These include addressing equity concerns from patient perspectives, incorporating health equity considerations into economic analyses, consideration of access and its impact, and ensuring transparency in the decision-making process. Additionally, there is a need for a standardized health equity framework and increased collaboration to improve data generation relevant to HTA evaluations.
Figure. Potential recommendations based on excluded domains from the framework5
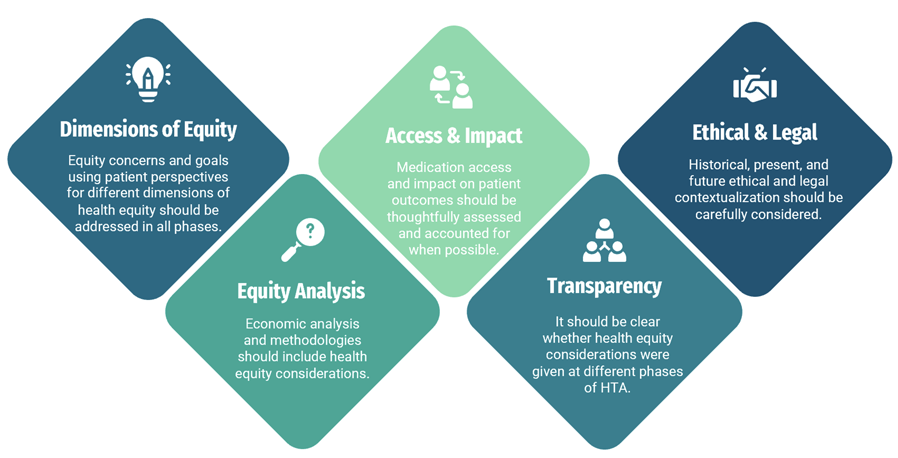
It is important to note that this study may not have reflected recent advances in health equity in HTAs, as health equity improvements more recently gained rapid traction due to the COVID-19 pandemic, highlighting stark health disparities globally.10
"There is a need for a standardized health equity framework and increased collaboration to improve data generation relevant to HTA evaluations."
More contemporary guidance was published by ICER in 202311 and CADTH in 2019.12 In this guidance, ICER established methods for US HTA with the objective of ensuring health equity gaps are ameliorated. The main recommendations included the following: (a) HTA bodies’ direct engagement with patients and patient groups during scoping to better understand the experiences and perspectives of the potential impact of the intervention under review, (b) setting a minimum threshold for adequate representation of racial and ethnic populations in clinical trials, (c) deterrence from computing cost-effectiveness estimates for subpopulations determined solely based on sociodemographic status, (d) encouragement to integrate thoughtful consideration of social values over quantitative equity-informative economic evaluation as a substitute, and (e) implementation of the process to identify healthcare structural changes necessary to ensure that disparities aren’t worsened with the introduction.11
The CADTH framework centers around patient engagement only and recommends the following:
(a) engage patients, families, and patient groups to enhance the quality and applicability of the evaluations and
(b) ensure that those affected by the HTA may actively contribute to the assessment. This framework is outlined using rationale and values (relevance, fairness, equity, legitimacy, capacity building), mechanisms of involvement, and diverse stakeholder involvement.12,13
Conclusion
The study underscores the gaps and lack of consensus on health equity incorporation within HTA, particularly in diseases with high unmet needs like cystic fibrosis. The findings may highlight the importance of collaboration to improve health equity-related data generation and standardization in HTA guidance to improve HTAs globally. Despite limitations of this work, such as language restrictions, public availability of assessments, and disease-specific focus, the study provides valuable insights for enhancing health equity integration in HTA processes.
Health equity-focused HTA helps identify disparities in access, outcomes, and costs, particularly for marginalized or underserved populations, further addressing health inequities that haven’t been fully uncovered and promoting social justice. The inclusion of health equity leads to better informed, ethical, and optimal decision making and resource allocation. Finally, these transparent efforts would assist in building connections in communities with historic mistrust in healthcare systems and prevent any unintended impact of introducing an intervention that widens health disparities.14
"Health equity-focused HTA helps identify disparities in access, outcomes, and costs, particularly for marginalized or underserved populations."
All stakeholders in the healthcare ecosystem should commit to action to ensure progress, albeit incrementally, towards optimal implementation of health equity considerations in HTA. Overall, by addressing existing gaps and implementing recommendations, stakeholders can promote fair and equitable access to healthcare technologies, ultimately improving health outcomes for all populations.
References
- Abersone I. Efficiency versus health equity in health technology decisions. Value & Outcomes Spotlight. 2022;8(4). https://www.ispor.org/publications/journals/value-outcomes-spotlight/vos-archives/issue/view/efficiency-versus-health-equity-in-hta/efficiency-versus-health-equity-in-health-technology-decisions
- O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. Jun 2020;36(3):187-190. https://pubmed.ncbi.nlm.nih.gov/32398176/
- Zhang M, Bao Y, Lang Y, et al. What is value in health and healthcare? a systematic literature review of value assessment frameworks. Value Health. 2022;25(2):302-317. https://www.ispor.org/publications/journals/value-in-health/abstract/Volume-25--Issue-2/What-Is-Value-in-Health-and-Healthcare--A-Systematic-Literature-Review-of-Value-Assessment-Frameworks
- van der Wilt GJ, Oortwijn W; VALIDATE-HTA Consortium. Health technology assessment: a matter of facts and values. Int J Technol Assess Health Care. 2022;38(1):e53. https://pubmed.ncbi.nlm.nih.gov/35950525/
- Benkhalti M, Espinoza M, Cookson R, Welch V, Tugwell P, Dagenais P. Development of a checklist to guide equity considerations in health technology assessment. Int J Technol Assess Health Care. 2021;37:e17. https://pubmed.ncbi.nlm.nih.gov/33491618/
- CADTH Canadian Drug Expert Committee. CADTH Canadian Drug Expert Committee Recommendation SR0559 Orkambi. CADTH Common Drug Review. October 4, 2018. Accessed June 13, 2023. https://www.cadth.ca/sites/default/files/cdr/complete/SR0559 Orkambi - CDEC Final Recommendation October 4%2C 2018.pdf
- Institute for Clinical and Economic Review. Modulator Treatments for Cystic Fibrosis: Effectiveness and Value. ICER Assessments. April 27, 2020. Accessed June 13, 2023. https://icer.org/wp-content/uploads/2020/08/ICER_CF_Evidence_Report_042720.pdf
- Committee for Medicinal Products for Human Use. Assessment Report: Kaftrio. European Medicines Agency CHMP Assessment Reports. July 10, 2020. Accessed June 13, 2023. https://www.ema.europa.eu/en/documents/assessment-report/kaftrio-epar-public-assessment-report_en.pdf
- Lee LY, Sheth ST, Valliant SN, Khan ZM. Review and evaluation of health equity considerations in health technology assessments in cystic fibrosis. Value Health. 2023;26(12 suppl):S257. https://www.valueinhealthjournal.com/article/S1098-3015(23)04464-9/fulltext
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(15):560-565. https://pubmed.ncbi.nlm.nih.gov/33857068/
- Advancing Health Technology Assessment Methods That Support Health Equity. 2023. Accessed March 13, 2024. https://icer.org/wp-content/uploads/2022/07/ICER_Advancing-Health-Technology-Assessment-Methods-that-Support-Health-Equity_040523.pdf
- CADTH Framework for Patient Engagement in Health Technology Assessment | CADTH. www.cadth.ca. Accessed June 12, 2023 https://www.cadth.ca/cadth-framework-patient-engagement-health-technology-assessment-0
- HTAi Interest Group for Patient and Citizen Involvement in HTA (PCIG). Values and standards for patient involvement in HTA. 2014. Accessed December 17, 2018. https://past.htai.org/interest-groups/pcig/values-and-standards/
- Husein F. Equity in health technology assessment. Can J Health Technol. 2023;3(10). https://www.canjhealthtechnol.ca/index.php/cjht/article/view/VP0003/1609

