Improving Patient Access to Medicines Targeting Early Stage Cancers in Europe
Charlotte Capdevila, MPhil, BS, Charles River Associates, London, England, UK; Oriana Ciani, PhD, MSc, BSc, SDA Bocconi School of Management, Milan, Italy; Michele Pistollato, PhD, Charles River Associates, London, England, UK; Ramiro Gilardino, MD, MSc, MSD Innovation & Development, GmbH, Zurich, Switzerland; Tilman Krueger, PhD, MSD Sharp & Dohme GmbH, Munich, Germany
Introduction
Timely diagnosis and prompt surgical treatment in early stages of cancer can enhance patient outcomes. Yet long-term studies indicate a higher risk of recurrence in some patients without the aid of (neo)adjuvant therapies.1 Development of medicines targeting early stage cancers (ESC) has increased in recent years and is expected to continue increasing, bringing significant benefits for patients and healthcare systems. However, there remain important barriers to patient access, including difficulties in recognizing the long-term benefits of those medicines or ineligibility because of delayed diagnosis and disease progression.
This analysis focuses on European countries and aims to provide a comprehensive overview of the benefits of innovative medicines for ESC, assess current challenges to patient access to such treatments, and identify key policy solutions to overcome those challenges.
Clinical and economic benefits of treating cancer early
Innovative medicines targeting ESC are associated with many clinical benefits and could improve quality of life through improving cure rates and decreasing/reducing the risk of relapse and cancer-related symptoms. In early stage non-small cell lung cancer (NSCLC), perioperative immune checkpoint inhibitor therapy has demonstrated a significant improvement in 24-month event-free survival rates from 40.6% to 62.4%.2 Similarly, in early stage triple-negative breast cancer (TNBC), combining immune checkpoint inhibitor therapy with chemotherapy has been shown to improve event-free survival at 36 months from 76.8% to 84.5% compared to chemotherapy alone.3 These therapies also improve surgical outcomes by increasing the success rate of tumor removal and reducing post-surgery complications. Furthermore, they have the potential to alleviate the mental health burden on patients by reducing the risk of tumor recurrence.4
Certain ESC-targeted therapies may be considered cost-effective or even cost-saving in the long-term. By aiming to prevent cancer recurrence, these medicines help reduce the need for higher-cost late-line treatments, resulting in overall cost savings. Studies in the United Kingdom have shown that treatment costs in breast, colorectal, and prostate cancers increase with disease stage and conclude that earlier stage diagnosis is associated with larger cost savings (Figure 1).5 In Germany, mean direct medical costs associated with breast cancer are twice as expensive for patients with stage IV compared to stage I.6 Another study shows that introducing immune checkpoint inhibitors in early stage melanoma, renal cell carcinoma, and TNBC helps avoid about 30% of active treatments in the metastatic setting over 10 years. Finally, use of immune checkpoint inhibitors in ESC have been demonstrated to prevent the need for neoadjuvant chemotherapy.7
Figure 1: Mean overall treatment costs per patient by stage in breast and colon cancers in the United Kingdom (adapted from Wills L, et al).5
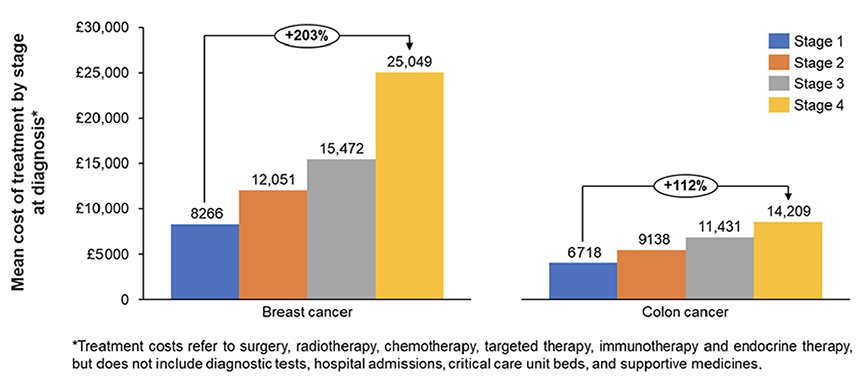
In addition to cancer care cost savings, medicines targeting ESC bring impactful economic advantages as they lead to a decreased need for non-oncology treatments and reduced economic costs associated with lost productivity. Psychological disorders affect up to 25% of cancer patients and double annual healthcare costs for those affected. Studies also show the psychological burden on families (eg, partners and children of patients with cervical cancer have 30-40% increased risk of mental disorders). Medicines targeting ESC may deliver psychological benefits to patients and their families by reducing anxiety and psychological stress caused by the risk of relapses, therefore improving quality of life and reducing nononcology treatment costs.8
"Development of medicines targeting early stage cancers has increased in recent years and is expected to continue increasing, bringing significant benefits for patients and healthcare systems."
With early diagnosis of cancer being more likely to impact younger patients in their working years, and with increased incidence of cancer cases in younger age groups, earlier treatment allows them to resume work earlier. Unemployment doubles from 2 years after endometrial or cervical cancer diagnosis and onwards. Productivity loss also impacts caregivers and families, as about 15% of caregivers of lung cancer and breast cancer patients left the workforce post-diagnosis.9
Challenges to patient access to medicines targeting ESC
While the clinical and economic benefits of medicines targeting ESC are increasingly acknowledged, patient access to those treatments remains more limited. Comparison of reimbursement rates for medicines targeting ESC compared to all cancer treatments in Europe shows that medicines targeting ESC face substantial barriers in achieving reimbursement, with an average reimbursement rate of 62% for medicines targeting ESC in Europe, compared to 77% for all cancer medicines (Figure 2). Studies show that high challenges are faced by both neoadjuvant and adjuvant treatments.10
Figure 2: Reimbursement status of medicines approved for ESC indications by the EMA between 2015 and 2019 in ESC versus all cancer medicines across European countries (Source: CRA analysis of publicly available data).
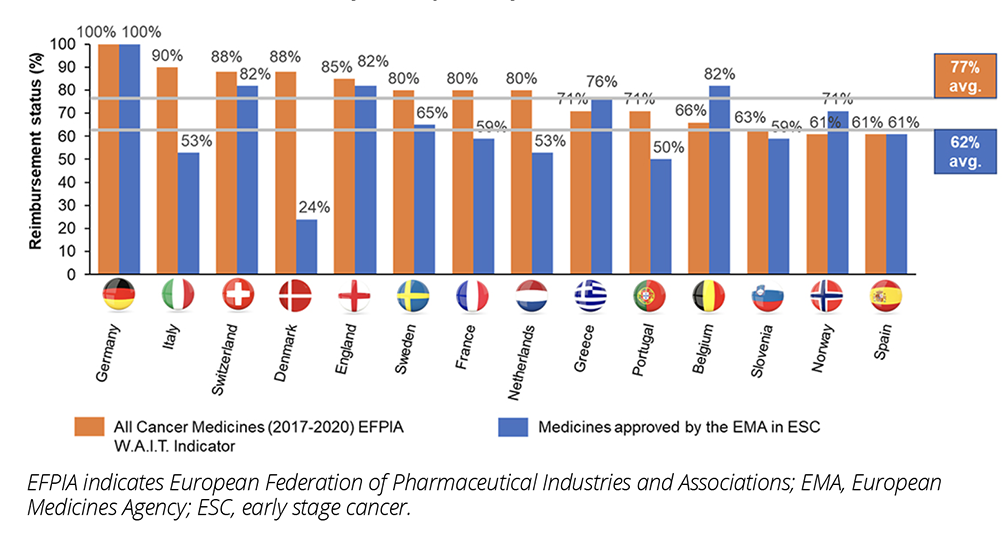
Despite the value drivers of treating ESC, challenges demonstrating cost-effectiveness are common, since HTA bodies and payers have traditionally considered median overall survival (OS) to be the “gold standard” clinical endpoint in oncology, but there are several issues affecting its use in novel treatments of ESC. Instead, disease-relevant endpoints are typically used and recognized as valuable by clinicians as they can better measure clinical and quality-of-life benefit in slow-progressing cancers, deal with the issue of confounding faced by OS for multiple consecutive lines of treatment, better capture the patient’s perspective, and shorten clinical trial durations. However, disease-relevant endpoints, and especially quality-of-life endpoints, are not universally accepted and views vary between stakeholder groups on the value of disease-relevant endpoints (eg, EUNetHTA21 guidelines state that while morbidity and health-related quality-of-life impact are valued, they are considered below mortality in the outcomes hierarchy).
In addition, affordability and underfunding of healthcare systems constitute other barriers to patient access to ESC. This manifests itself by deprioritization of treatments for ESC due to uncertainty around patient population size or long-term effectiveness. Consistency across countries on the type of data required supported by robust HTA methodological guidelines to be able to assess the long-term benefits of medicines targeting ESC (eg, long-term impact on patient survival, and local and distant metastasis-free survival), should be followed in practice.
"HTA bodies and payers have traditionally considered median overall survival to be the “gold standard” clinical endpoint in oncology, but there are several issues affecting its use in novel treatments of early stage cancers."
Once a medicine is reimbursed, other challenges can also hinder the use of medicines targeting ESC. Disease awareness from primary care practitioners, limited availability of screening programs to detect early disease, and low patient knowledge of these methods contribute to delayed diagnoses and cancer progression to later stages, leading to potential ineligibility for ESC treatments. Moreover, European/national guidelines recommending the use of medicines targeting ESC may not be updated frequently enough and/or widely adopted. Finally, fragmented healthcare systems and/or limited healthcare capacity can be a key issue (eg, patients not properly tracked in the system or not treated at the right time, low adoption of digitalized healthcare systems).
Solutions to improve patient access to medicines targeting ESC

A range of potential solutions is required to address multifactorial barriers to access to medicines targeting ESC (Figure 3). The systematic use of early dialog between manufacturers and HTA bodies/payers on disease-relevant endpoint evidence requirements is important to improve their acceptance and enabling timely patient access to treatments targeting ESC. Manufacturers and trade associations can work with regulators and payers and engage with clinicians and patient advocacy groups, to discuss approaches to reduce uncertainty related to clinical evidence and increase acceptance of disease-relevant endpoints. While the upcoming Joint Clinical Assessment (JCA) established by the EU HTA regulation may represent a first step toward greater harmonization of the type of evidence required, national appraisal and value assessment would remain at each country level. As there can be considerable variability in terms of endpoints acceptance and consequent patient access outcomes across European countries, there is an opportunity for local key opinion leaders to show their endorsement of disease-relevant endpoints in demonstrating the benefits of medicines in ESC.
Figure 3: Summary of key policy solutions to alleviate patient access barriers to medicines targeting ESC.
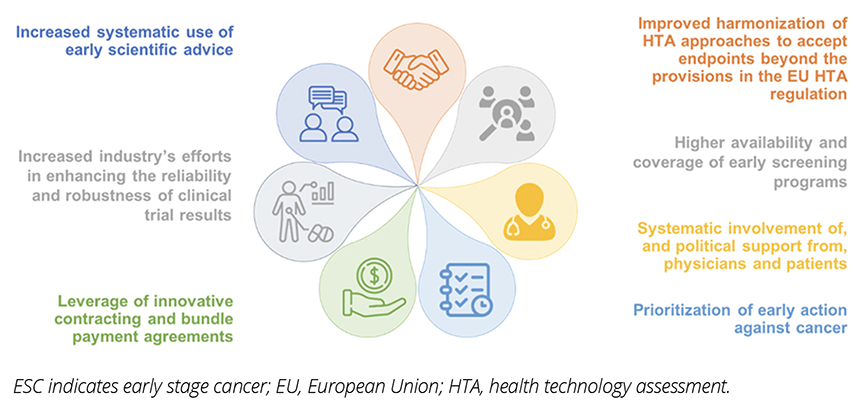
Where budgetary uncertainties linked to patient population size are a major concern for payers, volume-based contracting agreements (eg, price per volume contracting) or bundle payment models for both testing and treatment may be leveraged to alleviate concerns (eg, in France or Germany). It can also be useful for patient advocacy groups to support inclusion of ESC in national cancer control plans.
When looking to improve use of medicines targeting ESC, higher investment in screening and testing should first be considered to ensure as many cancers as possible are detected in early stages. This could be driven by the development and consideration of evidence of the clinical and economic benefits of early screening and testing. Uptake of screening may be improved through increased patient awareness, while involvement of physicians and patient advocacy groups in discussions supporting guidelines implementation could increase homogenization of higher quality-controlled practices in the real world and reduction of inequal access.
References
1. Cardoso R, Guo F, Heisser, et al. Overall and stage-specific survival of patients with screen-detected colorectal cancer in European countries: a population-based study in 9 countries. Lancet Reg Health Eu. 2022;21:100458. doi: 10.1016/j.lanepe.2022.100458
2. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early stage non–small-cell lung cancer. N Engl J Medi 2023;389:491-503. doi: 10.1056/NEJMoa2302983
3. Schmid P, Cortes J, Dent R, et al. Event-free Survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556-567. doi: 10.1056/NEJMoa2112651.PMID: 35139274.
4. Chang WH, Lai AG. Cumulative burden of psychiatric disorders and self-harm across 26 adult cancers. Nat Med. 28, 860-870.
doi: 10.1038/s41591-022-01740-3
5. Wills L, Nagarwalla D, Pearson C, et al. Estimating surgery, radiotherapy and systemic anti-cancer therapy treatment costs for cancer patients by stage at diagnosis. Eur J Health Econ. 2024;25(5):763-774. doi: 10.1007/s10198-023-01623-5
6. Khan SA, Hernandez-Villafuerte K, Hernandez D, Schlander M. Estimation of the stage-wise costs of breast cancer in Germany using a modeling approach. Front Public Health. 2023;6;10:946544. doi: 10.3389/fpubh.2022.946544.
7. Huang M, Fasching PA, Haiderali A, et al. Cost-effectiveness of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant single-agent pembrolizumab for high-risk early stage triple-negative breast cancer in the United States. Adv Ther. 2024;40, 1153–1170. doi: 10.1007/s12325-022-02365-1
8. Sundström K, Bencina G, Andersson M, Salomonsson S, Fang F, Wang J. Mental and socioeconomic burden in co-parents and children of patients with endometrial and cervical cancer: a Swedish population-based study [abstract]. Ann Oncol. 2023;34(suppl 2):S539. doi: 10.1007/s12325-022-02365-1
9. May SG. The impact of a cancer diagnosis on worker productivity: results from a survey of cancer patients and caregivers. J Clin Oncol. 2020;38(5 Suppl):144. DOI:10.1200/JCO.2020.38.29_suppl.144
10. Capdevila C, Pistollato M, Krueger T,
Roediger A, Gilardino R. Treating early often comes too late: ensuring patient access to early cancer detection and treatment in Europe. Presented at ISPOR Europe 2023; November 12-15, 2023; Copenhagen, Denmark. Value Health. 2023;26(12 Suppl):S270. doi: 10.1016/j.jval.2023.09.1412
Acknowledgments: The authors would like to thank Stina Salomonsson, Demet Sönmez, Agnes Brandtmüller, Zsófia Mária Nagy-Erdei, and Alexander Roediger for their comments in draft version of the manuscript.

